A
Anonymous
Guest
Hi all.
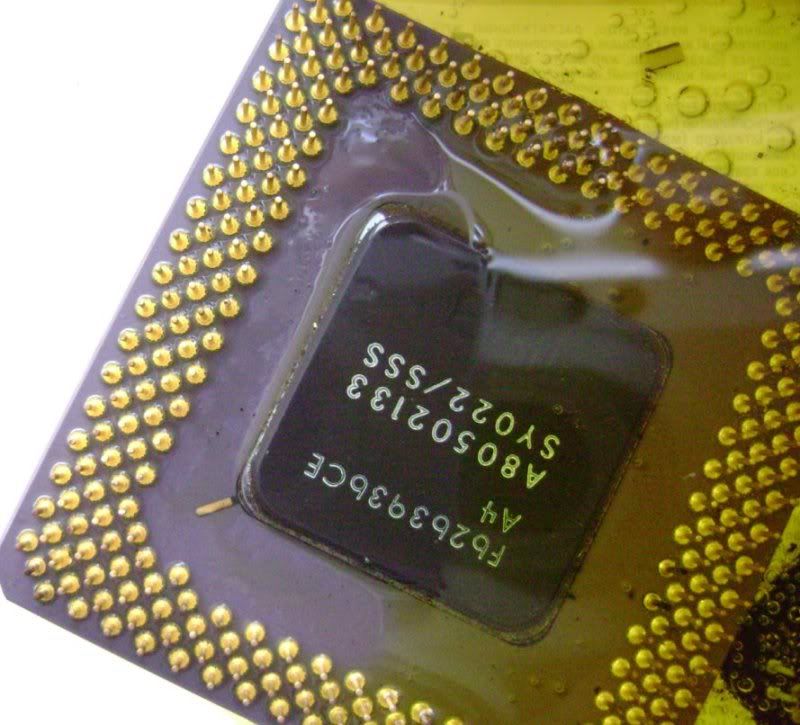
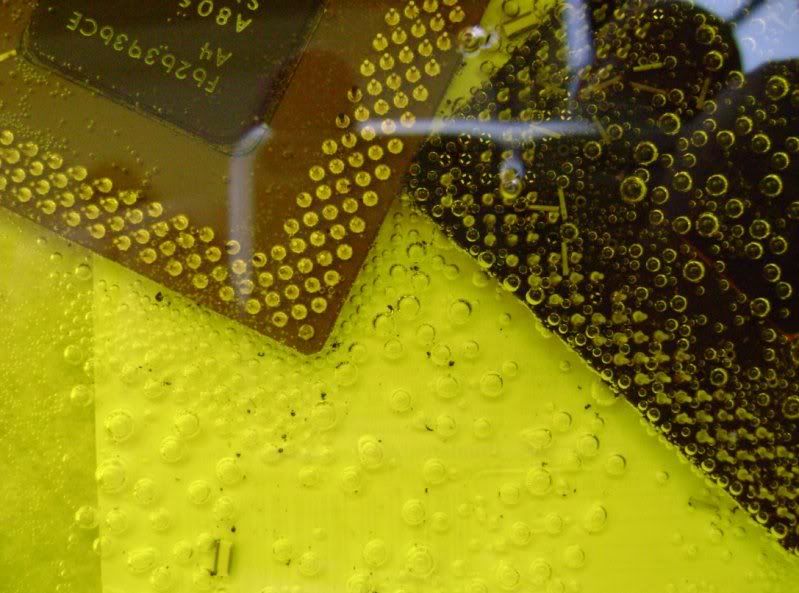
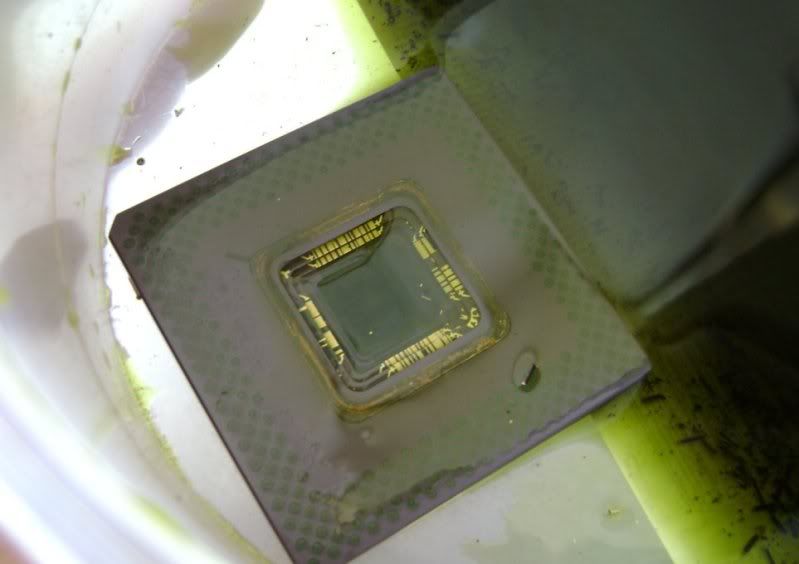
I am new in this forum and new in refining. I read most parts of the forum and deciced to try some thing. I put 2 cpu and put some 20 % hcl and some 3 % H2O2. ıts been nearly 2 hours. The liquid is now yellow. All the pins are staying on cpus. And i can see little bubbles around them. as i read the liquid should turn to green aor something. Can anyone teel me whats wrong ???
Thx for help.
I am new in this forum and new in refining. I read most parts of the forum and deciced to try some thing. I put 2 cpu and put some 20 % hcl and some 3 % H2O2. ıts been nearly 2 hours. The liquid is now yellow. All the pins are staying on cpus. And i can see little bubbles around them. as i read the liquid should turn to green aor something. Can anyone teel me whats wrong ???
Thx for help.