All,
There seems to be a lot of confusion on the various acid mixtures used in the different reactions. I've put together this quick list of reaction mixes. I know it's incomplete so I'll be adding to this list soon.
Here's a quick reaction list:

Testing with Schwerter's Solution
Apply a drop of Schwerter's Solution to the test piece.
The color reaction of the solution with the metal will be as follows:
Brass - Dark Brown
Copper - Brown
Nickel - Blue
Palladium - None
Gold - None
Silver Pure - Bright Red
Silver .925 - Dark Red
Silver .800 - Brown
Silver .500 - Green
Lead - Yellow
Tin - Yellow

You can smelt to instructions
This list is not all inclusive. There are many more methods to dissolve gold and base metals.
Steve
There seems to be a lot of confusion on the various acid mixtures used in the different reactions. I've put together this quick list of reaction mixes. I know it's incomplete so I'll be adding to this list soon.
Here's a quick reaction list:

- AR= Aqua Regia = 1 part 70% Nitric Acid (added in small increments), 3 parts Muriatic Acid (some guys use 4 parts muriatic). Used to dissolve high karat gold, gold powder, gold foils, dissolves Platinum when hot. Excess nitric must be evaporated off or neutralized with Urea to pH 1 +/- 0.4, then drop gold with SMB.
- Hoke states 4 fluid ounces HCl + 1 fluid ounce HNO3 dissolves 1 troy ounce gold. This is equivalent to 3.8 mL HCl + .95 mL HNO3 per gram of gold.

- AR Recipe 2= Poor Man's AR = 8 oz Sodium Nitrate (added in small increments),(aka Subzero), 480 ml water, 960 ml Muriatic Acid plus heat. Used to dissolve high karat gold, gold powder, gold foils, dissolves Platinum when hot. Excess nitric must be evaporated off neutralized with Urea to pH 1 +/- 0.4, then drop gold with SMB. In lieu of urea, I prefer Sulfamic Acid used in the dry crystals form. Add the crystals until the solution no longer fizzes when stirred. When using dry sulfamic acid you will have a small amount of undissolved crystals in the bottom of the solution once all of the fizzing stops. These left over crystals can be dissolved by adding a little warm water. Many thanks to 4Metals for the sulfamic acid tip!
- The above mentioned recipe makes enough AR to dissolve 160 gm Pins or 32 oz of ceramic cpus.

- HCl-Cl= Clorox Method = 4 Parts Muriatic, 1 Part Clorox (added in small increments). Used to dissolve gold foils and powder. Drop gold with SMB, NO urea needed.

- AP= Acid Peroxide = 2 Parts Muriatic Acid, 1 Part 3% Hydrogen Peroxide. Dissolves base metals, slowly dissolves gold when heated. If gold is present drop with SMB, NO urea needed. An even better method of precipitating dissolved gold is too continue using the solution until it becomes saturated with copper, then the gold precipitates as fine black powder.

Acid Peroxide Must Read
- Dilute HNO3=Dilute Nitric Acid= 1 part water, 1 part 70% Nitric Acid. Used to inquart, dissolve base metals, dissolves palladium, and dissolves silver. Silver nitrate will stain skin blue which turns black in sunlight. Skin remains black for nearly 1 week.
- Hoke states 4-6 pounds of concentrated nitric acid dissolves 1 pound of base metals. 5 pounds of 70% nitric, 1.41 sp.gr. is 1610 mL =~ 0.425 gallons. This equates to 1610 mL / 454 gm =~ 3.55 mL per gram.
-
Lazersteve said:Nitric with Copper
The key equation for copper from wiki is:
Equation (1) :
Cu + 4 HNO3 → Cu(NO3)2 + 2 H2O + 2 NO2
Copper (Cu) has an atomic mass of 63.55 grams per mole
70% Nitric acid has 15.8 moles per liter of nitric acid (HNO3).
Using the ratios from the equation above:
15.8 / 4 = 3.95
Therefore 1 liter of 70% HNO3 is enough HNO3 to dissolve 3.95 times the mass of copper in equation(1):
3.95 x 63.55g =~ 251 g ( 3.98 mL of 70% HNO3 per gram of copper)
Nitric with SilverThe key equation for silver from wiki (Wiki Silver Nitrate) is:
Equation (2):
Ag + 2 HNO3 → AgNO3 + NO2 + H2O
Silver (Ag) has an atomic mass of 107.9 grams per mole
70% Nitric acid has 15.8 moles per liter of nitric acid (HNO3).
Using the ratios from the equation above:
15.8 / 2 = 7.9
Therefore 1 liter of 70% HNO3 is enough HNO3 to dissolve 7.9 times the mass of silver in equation(2):
7.9 x 107.9g =~ 852.4 g (1.17 mL of 70% HNO3 per gram of silver)
The above numbers are the theoretical figures, in practice you will find that you can get more life out of your nitric by :
a. Adding water to the reaction.
b. Covering the reaction vessel with condenser or watch glass (reflux).
c. Capturing the NOx (mixed nitrogen oxides) off gases.
d. Recycling the nitrate by products of the reaction.
You can use the examples above to determine the amount of nitric required to dissolve any metal by substituting the appropriate equation and molar mass of the metal into the formulas given. Please note that nitric will not attack all base metals, and even passivates some and their alloys (wiki nitric acid). - Cold Nitric Acid Recipe
- Dry Nitric Acid Recipe
- Distilled Nitric Acid Recipe
- H2SO4= Sulfuric Acid = 1 cup 96%+ Sulfuric, 1/8 tsp glycerin (optional). Used as electrolyte in electrolytic cell along with a small amount of glycerin. Effective on medium to large gold plated items. With specially designed anodes and cathodes large batches of smaller plated items can be processed. Not used for Gold filled or karat jewelry.
- Use until acid is saturated with black powder typically 12-24 hours of operation. Sink any floating black powder, let settle overnight, pour off bulk of acid for reuse, and dilute remaining acid with 4 parts water.


Cell Information
Black Powder from the Cell
- HCl= Muriatic Acid = 31.45% Hydrochloric Acid. Used in the crockpot method and for general cleanup of gold foils and powders. Dissolves base metals. Also used in making stannous chloride gold testing solution.
- SMB= Sodium Meta Bisulfite (aka Storm Precipitant) plus water=28.3 Grams Sodium Meta Bisulfite, 240 ml H2O. Used to drop gold from gold bearing solutions.
- Add 65 grams to 100 ml water for saturated solution. Add to pregnant solution until stannous chloride test on solution is negative for gold.
Here's a procedure that can be used to clean and dry the resulting gold powder:
Cleaning and drying Gold Powder
Colors of Borax in Dish
Details of Gold Drying process
- AuCl3= Auric Chloride= Term used to describe gold dissolved into solution. Typically imparts a golden yellow color to solutions. Stains skin and other organics purple.

- SnCl2= Stannous Chloride= Used to test solutions for precious metals. Made by dissolving metallic tin in hot muriatic acid. Loses strength when stored. Made by adding 1-2 grams of powdered tin to 30 mL of HCl. Heat until fizzing starts. A little extra undissolved tin powder helps the solution keep for longer. Keep air out of container when stored to extend life.
Positive color test as follows:
Purple/Black color is Gold in solution, the darker the spot the more Gold.
Yellow/Brown that turns to Blue-Green after 30 seconds indicates Palladium in solution, the darker the spot the more Palladium.
Orange/Brown color is Platinum in solution, the darker the spot the more Platinum.
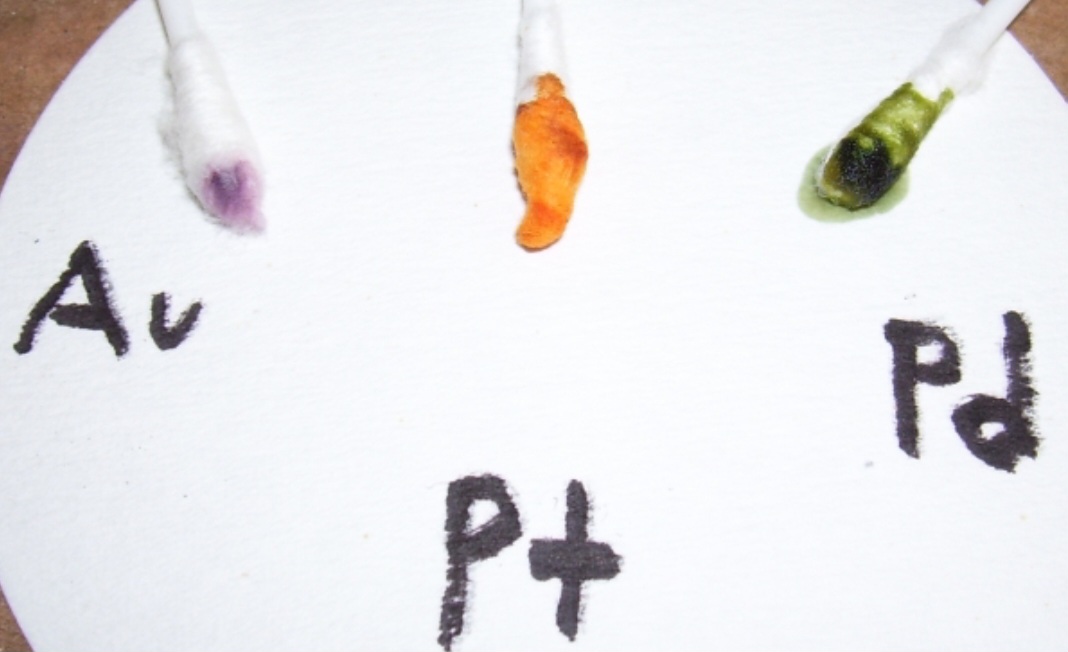
Stannous can be used to test for Rhodium in solution as described here:
Rhodium Test
Stannous Chloride False Positives
- (NH2)2CO= Urea= 8 oz Urea, 480 mL Water. Used to neutralize excess nitric acid in AR process before dropping gold with SMB. Add until solution doesn't fizz and pH reaches 1 +/- 0.4.
- H3NSO3= Sulfamic acid: Add dry crystals to pregnant solution with constant stirring to remove excess nitric acid via nitrous oxide. Once fizzing is no longer visible excess nitric acid is gone. Add enough warm water to dissolve any left over crystals in the bottom of the solution, filter 100% transparent, and precipitate your gold, platinum, or palladium. When large excess of nitric acid is present solution will foam vigorously and expand to 3 to 4 times it's original volume. Make sure your reaction vessel has enough head room for this rapid expansion. Sulfamic acid can also be used to test for excess nitric in solution: Add a pinch of sulfamic acid to the pregnant solution and observe the surface of the solution. If you see vigorous fizzing and foam around the area where the crystals entered the solution, you have excess nitric acid. Add sulfamic to pregnant solutions before any filtration to eliminate toxic NOx fumes when filtering. If you do not observe a vigorous fizzing and foam, your solution is free of nitric acid.
- AgCl= Silver Chloride= White precipitate that forms when silver is exposed to chlorine in solution. Turns purple in light, darkens further in sunlight. Solid by product of using AR on lower karat jewelry. Hazardous to melt due to fumes. Production of silver chloride should be avoided if possible. Can be converted to silver metal with lye and karo syrup or HCl and Al foil. Also can be converted using very dilute (10%) H2SO4 and an iron stirring rod. Do not let it dry out before conversion to silver metal.
For 1 tr.oz. of silver metal, about 41.5 grams of silver chloride, it takes about 20 grams of sodium hydroxide, 13.3 mL of light Karo syrup, and 133 mL of water. (Thanks GSP!)

- Precious Metals Precipitants Chart
- Inquartation= 3 parts base metal (Silver preferred) , 1 part Gold, Dissolved in hot dilute nitric acid. Powder/Honeycomb that remains is Gold and higher PGM's if present in source material. Left over liquid contains Silver, base metals, and Palladium if present in original alloy. Detailed Inquarting Process.
- Hoke states 4-6 pounds of concentrated nitric acid dissolves 1 pound of base metals. 5 pounds of 70% nitric, 1.41 sp.gr. is 1610 mL =~ 0.425 gallons. 1 gram of material requires 1610 / 454 =~ 3.55 mL HNO3 + 3.55 mL H2O.
Inquarted gold before acid processing:


Properly inquarted gold after acid treatment. - C12H26O3 =BDG or DBC= Butyl DiGlyme or DiButyl Carbitol = Organic solvent that combines with Auric Chloride and is insoluble in water based solutions. Separates from water based solutions as an upper phase (layer). After washing with dilute HCl gold is dropped as flakes using Oxalic Acid. Can be used on AR solution without neutralizing excess nitric acid.

- CH3C(NOH)C(NOH)CH3 =DMG or DimethylGlyoxime = Organic solid that combines with Palladium to form canary yellow crystals. Dissolve 1 gram in 25 mL of alcohol and add to 75 mL of hot water. Test with a single drop of DMG solution in a test tube sample of suspected palladium acid solution. In a minute or less you will see a yellow suspension of crystals if palladium is present.

- Conversion of Colored PGM salts to Sponge with Zinc Turnings
- Separating Mixed PGM Blacks
- Purifying Colored Palladium Salts
- Common Chemical Cross Reference
- HNO3 + K2Cr2O7 =Schwerter's Silver Test Solution = Add 20 grains of potassium dichromate to 30 mL of 35% nitric acid. Test by applying a single drop to clean silver surface.
Here is a test on sterling silver:

and A test of Pure silver:

Here's an alternate method for making the silver test solution:
- Dissolve Potassium Dichromate salt in 8mL of distilled water in a glass container. Add crystals until no more salt will dissolve in the liquid.
- Add 25mL of 70% Nitric Acid
- Store in a small bottle.
Testing with Schwerter's Solution
Apply a drop of Schwerter's Solution to the test piece.
The color reaction of the solution with the metal will be as follows:
Brass - Dark Brown
Copper - Brown
Nickel - Blue
Palladium - None
Gold - None
Silver Pure - Bright Red
Silver .925 - Dark Red
Silver .800 - Brown
Silver .500 - Green
Lead - Yellow
Tin - Yellow

You can smelt to instructions
This list is not all inclusive. There are many more methods to dissolve gold and base metals.
Steve
Last edited:










































































