- Joined
- Jun 22, 2023
- Messages
- 115
Hi Folks
1st let me say thanks for the wealth of information and intelligence of the community, and the shared resources.
So this one has me stumped, and maybe I'm just too dumb with a search engine, but, I have BUCKETS (40 to 50#'s worth) of gold-over-nickel ceramic dual inline package IC's. These also have interior gold bond wires.
They look like this
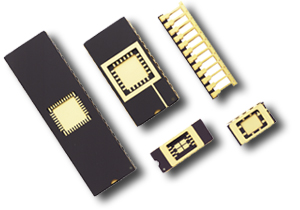
And have a gold-over-nickel cap on top. (I pulled the ancient MSDS / Package drawing to figure out the base metal. )
Now - I have read post after post about "cooking" the devices first and then crushing, but I don't think that's necessary in this case as the metals are NOT completely encapsulated once the cap is dissolved off.
But - my concern is that there is a huge % of the dissolved solution containing Nickel, which SMB precipitates out of AR along with Gold. Is there something I should use before SMB for gold to get the Nickel content down? Or rather than AR dissolving, go to a plating removal process instead (with AP or similar?)
First batch I was able to precipitate a nice brown foam that has been drying out for a bit now. I may have messed up and got into the spinning loop of death with excess NO2 as I had to use a LOT of SMB, but we'll see how that ends up. I did DeNox with Urea (I tried using BluDEF, maybe that's the issue) and never got any "black gold" precipitate but plenty of brown muck
So - I'm open for ideas. Thanks in advance and cheers!
1st let me say thanks for the wealth of information and intelligence of the community, and the shared resources.
So this one has me stumped, and maybe I'm just too dumb with a search engine, but, I have BUCKETS (40 to 50#'s worth) of gold-over-nickel ceramic dual inline package IC's. These also have interior gold bond wires.
They look like this

And have a gold-over-nickel cap on top. (I pulled the ancient MSDS / Package drawing to figure out the base metal. )
Now - I have read post after post about "cooking" the devices first and then crushing, but I don't think that's necessary in this case as the metals are NOT completely encapsulated once the cap is dissolved off.
But - my concern is that there is a huge % of the dissolved solution containing Nickel, which SMB precipitates out of AR along with Gold. Is there something I should use before SMB for gold to get the Nickel content down? Or rather than AR dissolving, go to a plating removal process instead (with AP or similar?)
First batch I was able to precipitate a nice brown foam that has been drying out for a bit now. I may have messed up and got into the spinning loop of death with excess NO2 as I had to use a LOT of SMB, but we'll see how that ends up. I did DeNox with Urea (I tried using BluDEF, maybe that's the issue) and never got any "black gold" precipitate but plenty of brown muck
So - I'm open for ideas. Thanks in advance and cheers!
Last edited by a moderator:


































































