You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
AR Process not completing
- Thread starter chawimac
- Start date

Help Support Gold Refining Forum:
This site may earn a commission from merchant affiliate
links, including eBay, Amazon, and others.
This may be something that one would want to ponder: When dissolving gold with some silver and getting the silver chloride precipitation if this action takes place in sufficient spectrum of light some of the exposed silver chloride is charged and prone to reduction to silver metal. Once reduced to metal the silver can occlude some gold by cementation. Once occluded, the gold is protected from aqua regia (by metallic silver) and remains in the silver/silver chloride mix. The remedy is obvious: Do the reaction either in the dark or under a red light. Back in 1968, we toned black and white photos with gold chloride in photography class in high school. Perhaps this may be the reason for gold remaining in your silver chloride mass. Dr. Poe
It is not uncommon for karat gold run in aqua regia without inquarting to leave gold behind in the chlorides. Do you know the assay of the silver in the starting bar? If the assay was much more than 7 1/2 % the silver chloride encapsulates some of the gold, the higher the silver assay, the more gold is retained in the chlorides.
I see production refining lots with silver up to 9% processed in aqua regia in large tumblers and the resulting chlorides can run as high as 1% in the silver bars. If they allow the silver to go higher the retained gold can exceed 5%. Personally I would rather refine in the light and not worry about the silver chlorides reducing in the light as there will always be some gold in the chlorides. The silver bars recovered from the aqua regia refining are perfect feedstock for a silver cell. Recover .999 fine silver and your gold will be in the slimes.
I see production refining lots with silver up to 9% processed in aqua regia in large tumblers and the resulting chlorides can run as high as 1% in the silver bars. If they allow the silver to go higher the retained gold can exceed 5%. Personally I would rather refine in the light and not worry about the silver chlorides reducing in the light as there will always be some gold in the chlorides. The silver bars recovered from the aqua regia refining are perfect feedstock for a silver cell. Recover .999 fine silver and your gold will be in the slimes.
Lobby
Well-known member
Many of you mention gold and silver precipitating from the AR solution due to the solution being too concentrated. Is this correct?
Silver always comes out of aqua regia as silver chloride. Gold will come out of aqua regia when it gets saturated with base metals which effectively cement out the precious metals. This is often confusing. If you are dissolving gold in acid along with base metals and you have a positive stannous reading, then after adding more scrap you have a negative stannous reading, the gold has dropped from the excessive base metals.
No big deal, simply decant the barren acid off the solids settled to the bottom and add more fresh acid.
Until you are accustomed to dealing with acids loaded with metals, it is a good idea to follow Harolds recipe to eliminate the base metals before cleaning up the gold.
No big deal, simply decant the barren acid off the solids settled to the bottom and add more fresh acid.
Until you are accustomed to dealing with acids loaded with metals, it is a good idea to follow Harolds recipe to eliminate the base metals before cleaning up the gold.
Lobby
Well-known member
Thanks.
The reason I ask is that I've had situations somewhat similar to the Original Poster where the "gold would not dissolve." Frustrating, to say the least.
This happened with the current batch. Several smallish pieces of metal wouldn't dissolve, yet when I added a button of 24k gold (as per Harold's suggestion), it reacted well. Hmmm.
I dropped the gold this afternoon. Will wash and melt tomorrow. The undissolved metals will be separately melted and analyzed. Let's see what I find out.
The reason I ask is that I've had situations somewhat similar to the Original Poster where the "gold would not dissolve." Frustrating, to say the least.
This happened with the current batch. Several smallish pieces of metal wouldn't dissolve, yet when I added a button of 24k gold (as per Harold's suggestion), it reacted well. Hmmm.
I dropped the gold this afternoon. Will wash and melt tomorrow. The undissolved metals will be separately melted and analyzed. Let's see what I find out.
Here is the post that I am writing with pics of my setup:
http://goldrefiningforum.com/phpBB3/viewtopic.php?f=61&t=13739
Thanks all for the help and hope the discussion follows the post as I have plenty of questions about equipment.
http://goldrefiningforum.com/phpBB3/viewtopic.php?f=61&t=13739
Thanks all for the help and hope the discussion follows the post as I have plenty of questions about equipment.
Lobby
Well-known member
Lobby said:Thanks.
The reason I ask is that I've had situations somewhat similar to the Original Poster where the "gold would not dissolve." Frustrating, to say the least.
This happened with the current batch. Several smallish pieces of metal wouldn't dissolve, yet when I added a button of 24k gold (as per Harold's suggestion), it reacted well. Hmmm.
I dropped the gold this afternoon. Will wash and melt tomorrow. The undissolved metals will be separately melted and analyzed. Let's see what I find out.
My results were quite interesting.
Here's a pic of the undissolved gold. Note the 24k piece to dissolve the unreacted AR (ala Harold).

I picked the undissolved gold pieces off the filter paper and melted them. I couldn't help but pick up a little AgCl, so I added a bit of soda ash to the flux to convert the AgCl to Ag.
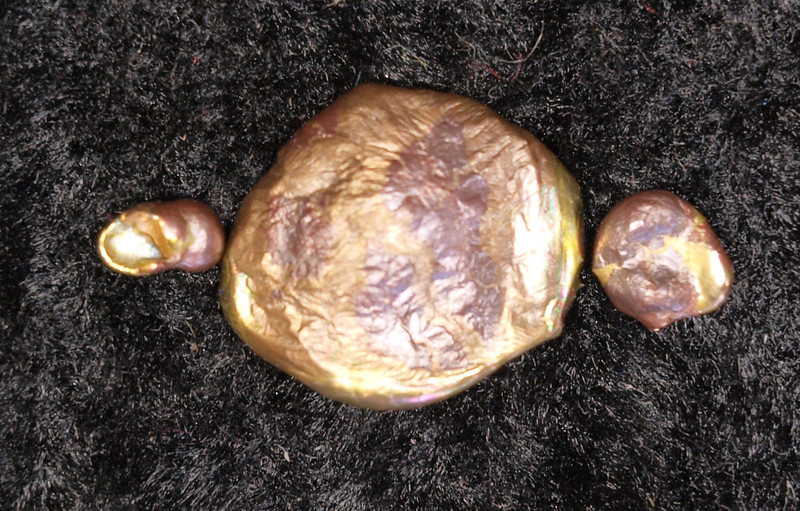

Total weight of the pieces = 1.4 g
31% gold, 69% silver, no Pt nor Pd (by XRF - thanks Dan Dement!)
This amounts to about 0.9% of the initial gold that was undissolved. Is a 1% undissolved rate typical?
The batch I'm working on right now also had some left over gold. (I haven't collected it yet). Looks like this is a systemic thing with me.
Thoughts?
Well the mix of silver to gold seems a little low for dissolution in nitric and that's where I suspect your problem may be, the nitric hasn't reacted with these pieces, they may also have been shielded from it and when then put into AR the silver forms a chloride coat which stops the gold been dissolved. A second treatment with hot nitric might dissolve those pieces but it would take time and if time is important I'd simply remove any pieces left after AR and put into the next melt.
Lobby
Well-known member
I had the same thing occur on the batch I'm currently working on: incomplete dissolution of the cornflakes, while a 24k nugget placed in the solution to finish off the nitric readily reacts.
I'm starting to think that a percent or so of inquarted metal not dissolving in AR is normal and I should just plan for it.
I'm starting to think that a percent or so of inquarted metal not dissolving in AR is normal and I should just plan for it.
Geo
Well-known member
i agree with Nick, passivization occurs when a coating of silver chloride stops the reaction. it could be the mix of metals that your using to inquart.
Lobby
Well-known member
Just regular old 925 sterling.
maynman1751
Well-known member
Are you sure that you are getting a thorough mixing of the silver and gold during the inquarting melt, stirring the molten metals with a carbon rod? You probably already know that if the metals are not mixed well it's possible for some of the shot to be too rich in gold to dissolve. Just something to think about!!!!
Lobby
Well-known member
Getting ready to start Batch 8:

Stirring rod at the ready. Gold underneath, silver on top.
:mrgreen:

Stirring rod at the ready. Gold underneath, silver on top.
:mrgreen:
- Joined
- Feb 25, 2007
- Messages
- 8,360
Most likely not a mystery at all. If you've read Hoke, you have a firm understanding of the effects of silver in gold alloy. I'll describe what it looks like, from which you may be able to draw a conclusion as to why your gold wouldn't dissolve.Lobby said:Several smallish pieces of metal wouldn't dissolve, yet when I added a button of 24k gold (as per Harold's suggestion), it reacted well. Hmmm.
When a piece of gold has excessive silver content (I would suggest greater than 10%, or less, if you're working with ambient temperatures), when you apply AR, the free gold on the surface readily dissolves, and traces of silver that are exposed convert to silver chloride. If the percentage of silver is low enough, the silver chloride is shed, exposing more metal beneath the surface from which it came. However, when the concentration level is too high, it continues to form, but does not shed. Instead, it forms a hard inert coating that prevents acid from contacting the metal beneath. Even prolonged boiling won't penetrate the surface, which, more or less, becomes impervious.
If you were to remove metal that has been so altered, you'd notice that it has a rather strange green/gray color. It can be processed by re-melting, adding a sufficient amount of silver (inquarted) so it can be parted successfully with dilute nitric, or it can be cleaned with ammonium hydroxide, then sent back to AR. However, if the pieces are large and thick enough, that isn't a solution, as the condition will repeat until all of the inner metal has been dissolved. It's a waste of time to not re-melt with silver.
Harold
Lobby
Well-known member
Harold_V said:Lobby said:Instead, it forms a hard inert coating that prevents acid from contacting the metal beneath. Even prolonged boiling won't penetrate the surface, which, more or less, becomes impervious.
Thanks so much, Harold.
Looking at the pic of the particles on the filter paper again, the hard silverish crust on the gold is evident.
The batch I completed yesterday had incomplete dissolution in AR also: about 1 gram out of a total of 61 grams of gold: it looks like I'm maintaining about 1% undissolved gold. I'll quit worrying about it and just recycle that gold from the filter papers in the subsequent batch.
Is this a common occurrence? Did you have this occur often with your AR steps?
- Joined
- Feb 25, 2007
- Messages
- 8,360
Not common, no, but it did occur occasionally. Generally when I didn't hit the desired 25% gold content when I did my inquarting. I had a "rule of thumb" that seemed to work quite well, which was to weigh the incoming gold scrap, then add 110% of the weight with scrap silver. The only time I deviated from that was if there was a showing of dental gold included, at which time I'd add more silver to offset the typically higher gold percentage. On rare occasion, the silver content might be too low, so the nitric digest was difficult, and occasionally incomplete. On such (rare) occasions, I would re-melt, adding more silver.Lobby said:Is this a common occurrence? Did you have this occur often with your AR steps?
I did not have the luxury of identifying each piece that was melted. It often came in in large volumes, and, because I processed individual lots, there was no need for me to assay. I had no good way of knowing the percentage of gold, so there was room for error when I'd inquart. Do keep in mind, inquartation will work through a wide range, however, so unless you err on the side of too little silver, the material will always part. It's just that the remaining gold may crumble, making separation of the fluids from the solids a little more difficult. A nuisance thing, really.
Harold
Lobby
Well-known member
Hmmm.
You must have normally received about 10k scrap. 110% of scrap gives about 25% gold, if the incoming scrap is close to 10k. But if it averages 14k (admittedly higher grade scrap), then I calc that closer to 190% of scrap weight with Ag is required.
Since this is the gold I buy at my scrap biz, I typically separate the various karat gold. I then calculate an "exact" inquartation amount. As I'm pretty sure I'm stirring well enough, I'm probably going to try "infifthing" :mrgreen: instead of inquarting for the next batch. It would be nice if I just didn't have to add another step to my processing.
You must have normally received about 10k scrap. 110% of scrap gives about 25% gold, if the incoming scrap is close to 10k. But if it averages 14k (admittedly higher grade scrap), then I calc that closer to 190% of scrap weight with Ag is required.
Since this is the gold I buy at my scrap biz, I typically separate the various karat gold. I then calculate an "exact" inquartation amount. As I'm pretty sure I'm stirring well enough, I'm probably going to try "infifthing" :mrgreen: instead of inquarting for the next batch. It would be nice if I just didn't have to add another step to my processing.
dtectr
Well-known member
Maybe I'm tired and my pain pills haven't kicked in yet, but G-zus !
Chawimac - if you can't afford the additional silver, [unless you were left it and your too expensive glassware by a rich old uncle], (yeah - I read the other thread) - congratulations on wasting money on equipment and then pissing off the only ones qualified to pluck your ass from the fire!)
Maybe you've refined several troy ounces of gold, (or pounds of silver, since it turns you on more) - I haven't yet but what I have managed to recover, refine and sell is thanks to the presentation of millenia of knowledge here at GRF; so when I see someone get in trouble by ignoring simple explanations of more complicated processes, then beg for help, then ignore sound, acknowledged direction, then insult the individuals who try to politely put his stupid ass back on track - I find that I am at a loss for words, for once.
My dad called it "spending a dollar to save 15¢". My grandfather said "Measure it twice and cut it (a board, in his case) once."
You have 2 options at this point, as I see it -
1. Continue on in the foolish course you've been pursuing, with the results you've been realizing, or
2. Accept the direction offered here in the spirit it was intended, and begin seeing the full potential of the stock you are processing. If that doesn't appeal to you, you could always send me your feedstock and let me process it, according to established procedures - who do YOU think will end up with more money at the end of the day?
Chawimac - if you can't afford the additional silver, [unless you were left it and your too expensive glassware by a rich old uncle], (yeah - I read the other thread) - congratulations on wasting money on equipment and then pissing off the only ones qualified to pluck your ass from the fire!)
Maybe you've refined several troy ounces of gold, (or pounds of silver, since it turns you on more) - I haven't yet but what I have managed to recover, refine and sell is thanks to the presentation of millenia of knowledge here at GRF; so when I see someone get in trouble by ignoring simple explanations of more complicated processes, then beg for help, then ignore sound, acknowledged direction, then insult the individuals who try to politely put his stupid ass back on track - I find that I am at a loss for words, for once.
My dad called it "spending a dollar to save 15¢". My grandfather said "Measure it twice and cut it (a board, in his case) once."
You have 2 options at this point, as I see it -
1. Continue on in the foolish course you've been pursuing, with the results you've been realizing, or
2. Accept the direction offered here in the spirit it was intended, and begin seeing the full potential of the stock you are processing. If that doesn't appeal to you, you could always send me your feedstock and let me process it, according to established procedures - who do YOU think will end up with more money at the end of the day?
Chawimac the point your missing when you say the big refiners don't use inquartation is that they use assays or xrf readings to make sure they don't have too much silver in the mix to cause the problems your having, the other point to remember is that that they need fast, very fast turnarounds of their materials and a little left over to be refined next time is no big thing to them compared to the huge amount of money that could be tied up for days on end. They run these processes day in day out so the material is rarely tied up for long if you are doing the same fine if not your wasting chemicals and time trying to copy them, you have been advised on the best course to take but seem reluctant to follow the advice, your choice but the people advising have been doing this successfully for years while you are struggling....does there seem to be any correlation between the two?



