The discussion around avoiding Stannic acid was the reason.I like this approach but instead of lye why not use HCl which will dissolve the tin leaving the gold
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
AuSn 80/20 alloy
- Thread starter Joe cocker
- Start date

Help Support Gold Refining Forum:
This site may earn a commission from merchant affiliate
links, including eBay, Amazon, and others.
Look at bold text, if you dissolve it like this, you have to use SMB or similar.Gold do not dissolve into HCl, Tin(Sn) do.
The mud will be the gold.
If you suspect more Tin is left.
Dissolve the powder in HCl Peroxide or HCl Bleach.
Do NOT use AR, because Tin and Nitric makes metastannic acid.
After dissolving, precipitate with SMB, wash and melt.
And this was the reason I asked if you understand the advice you are given.
HCl do not dissolve gold, HCl and Peroxide or chlorine do.
Metastannic acid is the result of Nitric (AR) and tin.
I believe there is a serious language barrier here, and he do not understand much of the advice he was given.The discussion around avoiding Stannic acid was the reason.
HCl and tin do not make metastannic acid.The discussion around avoiding Stannic acid was the reason.
There is no thing in the original post contradicting the use of Zinc, I'm sure Zinc was not even discussed before I mentioned it as an input to "inquarting", which has no place here.Read the original post for the answer.
There is no part of this alloy that will warrant inquarting. The easiest would be to go directly to HCl peroxide, since it already are in "powder" form.
I will only add the fact that zinc and tin do not mix very well. If you are familiar with Parkes process for recovering of silver from lead, you know that zinc is used to "extract" the silver from bulk of the molten lead - because zinc and lead do not mix.When we are at it, why "inquart" with tin? Why not Zinc if you have it readily available.
It dissolves even easier.
Similarly, tin and zinc do not mix very well and you could use "Parkes-like" process to scavenge silver out of molten tin with zinc - but only to some extent, it is not nearly as effective as with lead.
Zinc will alloy with gold persumably, but you will probably get two-phase alloy - AuZn phase and AuSn phase. Maybe they form some terenary mixture, but this is only guessing
If you allow the mixture to cool slowly, phases will start to separate and you get non-homogenous crystal phases = you could possibly create quite a mess
Inquarting with more tin and making the cornflake could work quite nicely with good strength HCl. Good surface area will be the key. Also, if you cannot get the last bit of tin out, I think it won´t be that bad - just bit of metastannic in AR workup of resulting sponge. Filters much more easily than whole 20% in the starting material
Also, inquarting and whole preparation will be much more easy when considering quite low melting point of tin.
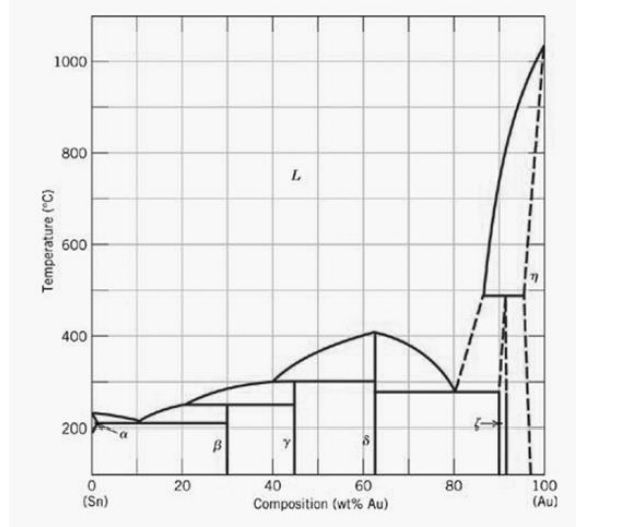
According to the binary phase diagram, somwhere near 300°C should be theoretically enough to create homogenous liquid phase with 25% gold. Personally I will go as high as 500°C and shot this into the ice water - very quick cooling could help retain the homogenity of the alloy.
Thanks Orvi.I will only add the fact that zinc and tin do not mix very well. If you are familiar with Parkes process for recovering of silver from lead, you know that zinc is used to "extract" the silver from bulk of the molten lead - because zinc and lead do not mix.
Similarly, tin and zinc do not mix very well and you could use "Parkes-like" process to scavenge silver out of molten tin with zinc - but only to some extent, it is not nearly as effective as with lead.
Zinc will alloy with gold persumably, but you will probably get two-phase alloy - AuZn phase and AuSn phase. Maybe they form some terenary mixture, but this is only guessingHowever, temperature will be the key point, as the mixing of the three components will be largely affected by temperature.
If you allow the mixture to cool slowly, phases will start to separate and you get non-homogenous crystal phases = you could possibly create quite a mess
Inquarting with more tin and making the cornflake could work quite nicely with good strength HCl. Good surface area will be the key. Also, if you cannot get the last bit of tin out, I think it won´t be that bad - just bit of metastannic in AR workup of resulting sponge. Filters much more easily than whole 20% in the starting material
Also, inquarting and whole preparation will be much more easy when considering quite low melting point of tin.

According to the binary phase diagram, somwhere near 300°C should be theoretically enough to create homogenous liquid phase with 25% gold. Personally I will go as high as 500°C and shot this into the ice water - very quick cooling could help retain the homogenity of the alloy.
It was not a serious suggestion, just a reaction to the proposal of inquarting. Totally unnesccessary for this issue. The solder was in "micro" whisker form according to the original poster, hence HCl-H2O2 will dissolve it quite fine. Problem solved.
There are plenty challenges within refining. We don't need to create more of them.
The comments of the OP also indicates that he do not neccessarily read the advice he is given, or may have a language issue that makes it hard for him to see it clearly.
He have not replied to this.
Yeah. I know.Thanks Orvi.
It was not a serious suggestion, just a reaction to the proposal of inquarting. Totally unnesccessary for this issue. The solder was in "micro" whisker form according to the original poster, hence HCl-H2O2 will dissolve it quite fine. Problem solved.
There are plenty challenges within refining. We don't need to create more of them.
The comments of the OP also indicates that he do not neccessarily read the advice he is given, or may have a language issue that makes it hard for him to see it clearly.
He have not replied to this.
But it is a good-to-know fact, and could be of interest for more people, if they intend to refine similar material somewhere in the future
Just to prevent unpleasant findings with expensive material.
I previously advised just to use regular AR for that relatively small quantity of material. Filtering will be slow, but doable on that scale.
I have not made metastannic acid to my knowledge, but guess there have been some present from time to time.
My feedstock has been quite suitable for HCl Peroxide so far. I just used the sledge hammer (AR) a few times.


My feedstock has been quite suitable for HCl Peroxide so far. I just used the sledge hammer (AR) a few times.
Last edited:
Completely correct, and also that was NOT the reason for the discussion - I misremembered the context.HCl and tin do not make metastannic acid.
The question about inquartation /w tin was in response to some conversation about various ways to deal with tin since the source material is said to be an alloy of tin/gold. I did some research and it looked like it's possible to get better dissolution of tin in lye than HCL. Note, this was a left-field question from me, and not at all posed as advice. Further, I thought the topic was sorted, and I'm not sure why it's brought up now.
What about aqua regia bath and then mud is washed with HCl you'll get fine goldUp up ! Hope someone will be able to separate this tin from the alloy !
Thanks
You do not want Aqua Regia and Tin in the same beaker together.What about aqua regia bath and then mud is washed with HCl you'll get fine gold
Nitric and Tin creates Metastannic acid, which is a nightmare to filter.



