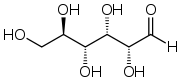
Butcher, that was a really detailed and exhaustive answer... sadly it's wrong and not what was asked. The question was in other words "To convert 2Ag
+ to 2Ag you need two electrons. One comes from C
6H
11O
6 that turns into C
6H
11O
7-+Na
+, where does the second electron come from?"
Well, maybe your answer wasn't totally wrong, but at least it contains an error.
butcher said:
2AgCl (s) + 3NaOH (aq) + C6H11O6 --> 2Ag(s) + 2NaCl (aq)+ C6H11O7 Na (aq)
Both sides contains only neutral compounds so obviously the electrons should balance out, but does the elements balance?
2AgCl + 3NaOH + C
6H
11O
6 --> 2Ag + 2NaCl + C
6H
11O
7Na
2 Ag on both sides, 2 Cl on both sides, 3 Na on both sides, 9 O on left but 7 on right, 14 H on left but 11 H on right, 6 C on both sides.
You have forgotten 3 H and 2 O on the right hand side, sounds like an H
2O and OH (Without charge! :shock: ).
I suspect that the equation is more like (Where the aldehyde group -H-(C=O) > turns into a carboxylic acid (-COOH)) and the Na is actually Na
+ associated with OH
-
2AgCl + 3NaOH + C
6H
11O
6 --> 2Ag + 2NaCl + C
6H
11O
7 + H
2O + NaOH
A second step could turn the carboxylic acid group (-COOH ) and the NaOH into (-COONa) and H
2O so the total equation is (changes in red)
2AgCl + 3NaOH + C
6H
11O
6 --> 2Ag + 2NaCl + C
6H
10O
7Na +
2H2O
The answer to the question is : The change in oxidation state of the 2Ag
+ reduced into 2Ag is powered by the aldehyde group is oxidized into carboxylic acid by an oxygen atom.
butcher said:
Those are moles of units of silver and moles of glucose...
It doesn't matter if we are talking about electrons and atoms or moles of electrons and atoms. The equations must balance in the end.
Disclaimer : I'm a mathematician and physicist so my chemical knowledge is nothing but a couple of google searches and wikipedia, but I can count and spot an error. So I'm standing by my statement that there is an error but my solution could be just as wrong or even worse. Time to let the chemists check my reasoning.
Göran