Lobby
Well-known member
Folks,
I'm on my 4th batch of gold refining. 1st two batches (run concurrently) had some problems, but made 99+% gold. 3rd batch (10k scrap only) was just about perfect. In all these 3 batches, I've had no problems during the gold drop step. Either with copperas or SMB.
I'm in the middle of batch 4 (96.1 g of 14k scrap = 56.1 g gold)
During the inquartation, I had a bit of a problem. I had a heck of a time seasoning the new, 5 inch melting dish I just bought. I just couldn't get an even coating of borax on the dish; areas just didn't want to take the borax.
So I put too much on the dish, I suppose.

The resulting cornflakes contained some frozen borax on them (from dropping molten borax on top of the cornflakes in the water pot. You can see the purple borax specs in the pic.

I thought about remelting (especially since I searched through the forum and found a recipe for a better flux - thank folks!), but in the end, didn't. I used tooth cleaning pics the dentists use to clean the cornflakes. I got most of the borax off.

The HNO3 dissolution went uneventfully. After the reaction was ended, I poured off the solution and added fresh, concentrated HNO3 to the pot, enough to cover the cornflakes.

Boiled for a few minutes. Got some red fumes, but they cleared, so I turned off the hot plate and let the solution cool.
I then started the AR step. I added about 225 mls of conc HCl to the beaker and started heating.

Note the 100 ml beaker containing conc HNO3 with the plastic pipette. I normally add HNO3 in about 5 ml steps, to not overshoot.
The last HNO3 addition was 2 mls. With the hot plate on, the solution was slightly boiling, but no red fumes in the gas phase. I assumed the rxn was over, and I was merely boiling the solution.
Here I am about to filter the AR solution. Note how dark it is. Note the filter paper with the plastic tweezers on the right. Those pieces are unreacted gold from the AR solution.

Well, the SMB drop was horrible. I added a solution containing about 80 grams SMB (same SMB as the good Batch 3), dropped gold, but solution still showed gold (SnCl2). As I had no more solution, I started adding SMB powder. Every time I did, the solution would bubble and give off sulfur gasses. Yuck. I must have added about 100 grams more of SMB. Sigh. Finally, my solution showed clear of gold. I let it stand overnight. In the AM, it still showed clear of gold.
But big problem: I had white power mixed with the gold in the beaker. Obviously unused SMB, although perhaps some other sodium salt.
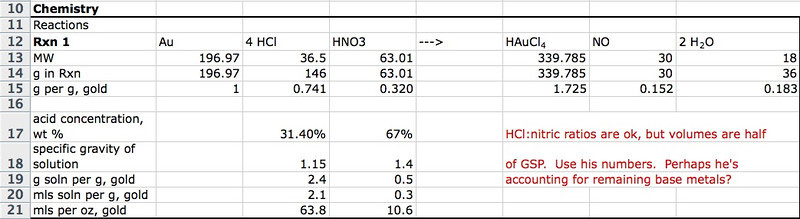
What did I do? I started washing the solution with water. 100 mls at a time, vigorous stirring, then settle for an hour or so. I think I did about 6 washes today. I'm about to do another.
And will continue washing tomorrow. The gold isn't free flowing. It feels, with the stirring rod, sorta muddy.
Questions:
- what the heck happened? Would remaining borax flux affect the AR reaction like that?
- can ya'll recommend a washing procedure to insure I remove the remaining SMB salts? I've been cautious about adding any acids to the mix.
Thanks.
I'm on my 4th batch of gold refining. 1st two batches (run concurrently) had some problems, but made 99+% gold. 3rd batch (10k scrap only) was just about perfect. In all these 3 batches, I've had no problems during the gold drop step. Either with copperas or SMB.
I'm in the middle of batch 4 (96.1 g of 14k scrap = 56.1 g gold)
During the inquartation, I had a bit of a problem. I had a heck of a time seasoning the new, 5 inch melting dish I just bought. I just couldn't get an even coating of borax on the dish; areas just didn't want to take the borax.
So I put too much on the dish, I suppose.

The resulting cornflakes contained some frozen borax on them (from dropping molten borax on top of the cornflakes in the water pot. You can see the purple borax specs in the pic.

I thought about remelting (especially since I searched through the forum and found a recipe for a better flux - thank folks!), but in the end, didn't. I used tooth cleaning pics the dentists use to clean the cornflakes. I got most of the borax off.

The HNO3 dissolution went uneventfully. After the reaction was ended, I poured off the solution and added fresh, concentrated HNO3 to the pot, enough to cover the cornflakes.

Boiled for a few minutes. Got some red fumes, but they cleared, so I turned off the hot plate and let the solution cool.
I then started the AR step. I added about 225 mls of conc HCl to the beaker and started heating.

Note the 100 ml beaker containing conc HNO3 with the plastic pipette. I normally add HNO3 in about 5 ml steps, to not overshoot.
The last HNO3 addition was 2 mls. With the hot plate on, the solution was slightly boiling, but no red fumes in the gas phase. I assumed the rxn was over, and I was merely boiling the solution.
Here I am about to filter the AR solution. Note how dark it is. Note the filter paper with the plastic tweezers on the right. Those pieces are unreacted gold from the AR solution.

Well, the SMB drop was horrible. I added a solution containing about 80 grams SMB (same SMB as the good Batch 3), dropped gold, but solution still showed gold (SnCl2). As I had no more solution, I started adding SMB powder. Every time I did, the solution would bubble and give off sulfur gasses. Yuck. I must have added about 100 grams more of SMB. Sigh. Finally, my solution showed clear of gold. I let it stand overnight. In the AM, it still showed clear of gold.
But big problem: I had white power mixed with the gold in the beaker. Obviously unused SMB, although perhaps some other sodium salt.
What did I do? I started washing the solution with water. 100 mls at a time, vigorous stirring, then settle for an hour or so. I think I did about 6 washes today. I'm about to do another.
And will continue washing tomorrow. The gold isn't free flowing. It feels, with the stirring rod, sorta muddy.
Questions:
- what the heck happened? Would remaining borax flux affect the AR reaction like that?
- can ya'll recommend a washing procedure to insure I remove the remaining SMB salts? I've been cautious about adding any acids to the mix.
Thanks.