So i have been using pliers to remove gold from stainless steel. The gold is welded on as u can see the back of one. Was looking for a better way to remove the flatter ones. When i bend it there is no lip to catch with utility knife or cutters. Any ideas would be appreciated. I was thinking of grinding gold off each one but that seems tough to do.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Removing gold from stainless steel jewelry
- Thread starter Rednight
- Start date

Help Support Gold Refining Forum:
This site may earn a commission from merchant affiliate
links, including eBay, Amazon, and others.
lazersteve
Well-known member
I run these in 20# batches using 50/50 nitric acid soak for 24 hours at room temperature. The karat gold logos, icons, flags, etc all easily separate with a slight scrape afterwards.
Treat the resulting material after removing the stainless like any other karat alloy.
The largest lot of them I've ever run was 200#s over a one month period.
Keep an eye out for the ones that are all epoxy or plated icons, they don't separate the same so be sure to sort them out.
Steve
Treat the resulting material after removing the stainless like any other karat alloy.
The largest lot of them I've ever run was 200#s over a one month period.
Keep an eye out for the ones that are all epoxy or plated icons, they don't separate the same so be sure to sort them out.
Steve
I never worked with acid so 50% water and nitric acid. I can buy that on Amazon? Let it sit in a
glass for 24 hours? To make sure I want to have the gold leftover. So after 24 hours, i can rinse with water? Also, can I let it soak in my garage? What's safe?
Thanks
glass for 24 hours? To make sure I want to have the gold leftover. So after 24 hours, i can rinse with water? Also, can I let it soak in my garage? What's safe?
Thanks
Golddigger76
Well-known member
Since you are new to recovery and refining slow down and learn how to first so you don't hurt yourself or anyone else who would or could be in the vicinity of your work area !!!!!
That gold is not going anywhere so don't be the next person to be asking how to fix a toxic mess because you didn't take the time to learn and plan how to recover and refine your gold.
Re-read lazersteve's post immediately above yours, any recommendations from him is informative and spot on.
Yes let them soak for 24 hrs then the gold should easily be dislodged from the stainless steel and do not use any acids in your garage unless you have a fume hood.
You can do the work outside where the fumes will blow away from your or your neighbors.
Do yourself a huge favor and read on this forum what equipment you need to protect yourself from acids, the fumes will burn your lungs, eyes, skin and will rust or corrode all metals in your garage if you don't have proper ventilation.
You can search the forums for almost anything you will need to know about safety, the equipment, procedures and chemicals you need to refine your gold or any precious metals.
If there's something you can not find by searching asking for help will be best.
That gold is not going anywhere so don't be the next person to be asking how to fix a toxic mess because you didn't take the time to learn and plan how to recover and refine your gold.
Re-read lazersteve's post immediately above yours, any recommendations from him is informative and spot on.
Yes let them soak for 24 hrs then the gold should easily be dislodged from the stainless steel and do not use any acids in your garage unless you have a fume hood.
You can do the work outside where the fumes will blow away from your or your neighbors.
Do yourself a huge favor and read on this forum what equipment you need to protect yourself from acids, the fumes will burn your lungs, eyes, skin and will rust or corrode all metals in your garage if you don't have proper ventilation.
You can search the forums for almost anything you will need to know about safety, the equipment, procedures and chemicals you need to refine your gold or any precious metals.
If there's something you can not find by searching asking for help will be best.
Since you did not answer my question on what to do with the Gold, and now are moving in the direction of chemical refining.I never worked with acid so 50% water and nitric acid. I can buy that on Amazon? Let it sit in a
glass for 24 hours? To make sure I want to have the gold leftover. So after 24 hours, i can rinse with water? Also, can I let it soak in my garage? What's safe?
Thanks
Here is what you need to know before starting the path to refining.
We ask our new members to do 3 things.
1. Read C.M. Hokes book on refining jewelers scrap, it gives an easy introduction to the most important chemistry regarding refining.
It is free here on the forum: Screen Readable Copy of Hoke's Book
2. Then read the safety section of the forum: Safety
3. And then read about "Dealing with waste" in the forum: Dealing with Waste
Suggested reading: The Library
Forum rules : https://goldrefiningforum.com/threads/board-policy-this-should-be-read-by-everyone.4646/
lazersteve
Well-known member
I've attached an assortment of photos below demonstrating a small fraction of the work I've done with the Itailian Charm Stainless Steel Bracelets brought up in the above post.


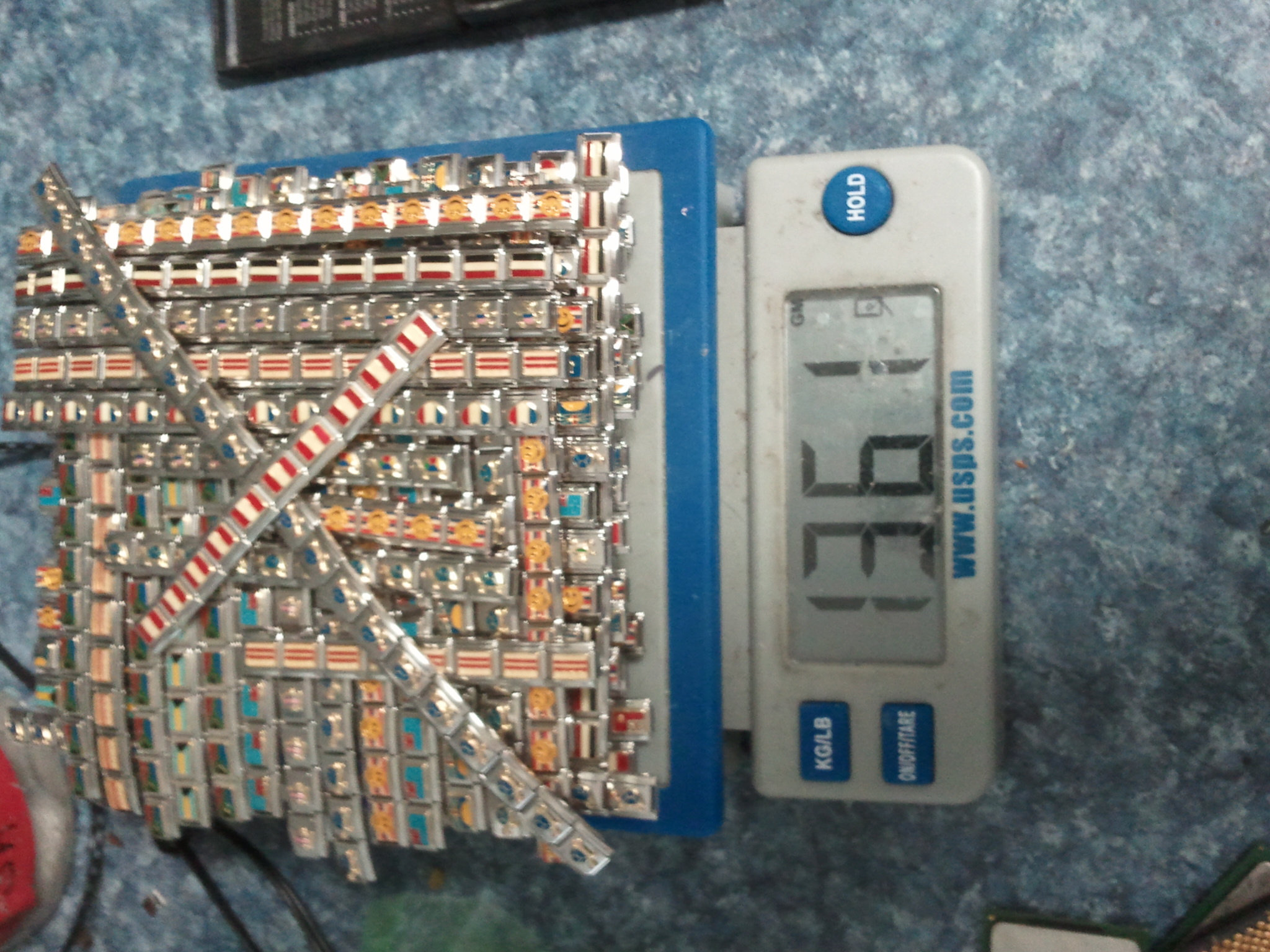





The above photos are of actual batches of these that I have run over the years.
A typical 20-30 pound batch of these is processed in less than 1 week with proper technique and chemical resources.
Steve








The above photos are of actual batches of these that I have run over the years.
A typical 20-30 pound batch of these is processed in less than 1 week with proper technique and chemical resources.
Steve
Last edited:
lazersteve
Well-known member
If you are not equipped to process these feel free to contact me atI never worked with acid so 50% water and nitric acid. I can buy that on Amazon? Let it sit in a
glass for 24 hours? To make sure I want to have the gold leftover. So after 24 hours, i can rinse with water? Also, can I let it soak in my garage? What's safe?
Thanks
[email protected]
about toll refining them for you, I have a fully equipped lab and first hand experience with processing this form of scrap in large quantities.
It's worth noting that there are a wide variety of these charms on the open market and many are fakes that contain no gold value as they are enameled stainless colored with epoxy resins. The ones in your photos appear to be the real ones.
I ran the batches I mentioned above for a customer that got them from Italy if the story I was told by my customer at the time is true.
Steve
Last edited:
I would try to throw everything into the molten lead in a low, wide crucible, about 500C (wood fire) is enough to remove the gilding (or small pieces of gold) in a few minutes, and steel doesn't form a solution with lead at all (solubility ~0). In a kilogram of molten lead, many tens of kilograms of your jewelry can be processed. There shouldn't be micro losses because no liquation of gold during rapid cooling (when extracting cleared hot metal bases from a mass of lead with a slotted spoon). Work under a hood with a filter, although at temperatures up to 800C, there is almost no lead vapor. After you can make cupellation in a magnesia or cement cupel or dissolve the lead-gold alloy in nitric acid. If gold was soldered with silver to steel, it will also be quickly removed by the lead.I recently purchased a large amount of charms that are used for making bracelets. They are stainless steel and 18k. They are not plated, just a small piece of gold on the front of ever piece. I would like to remove the gold from them, but every site I visited only talked about plating and not actual gold with some content. I would say each piece has about .1-.2 grams on it. I was thinking of just melting the piece and trying to separate it but I'm worried that it would bind it together. Please help. I'm a beginner. Thank you in advance.
Last edited:
Rednight has not been here since 2016 so he might not read it.I would try to throw everything into the molten lead in a low, wide crucible, about 500C (wood fire) is enough to remove the gilding (or small pieces of gold) in a few minutes, and steel doesn't form a solution with lead at all (solubility ~0). In a kilogram of molten lead, many tens of kilograms of your jewelry can be processed. There shouldn't be micro losses because no liquation of gold during rapid cooling (when extracting cleared hot metal bases from a mass of lead with a slotted spoon). Work under a hood with a filter, although at temperatures up to 800C, there is almost no lead vapor. After you can make cupellation in a magnesia or cement cupel or dissolve the lead-gold alloy in nitric acid. If gold was soldered with silver to steel, it will also be quickly removed by the lead.
Nice method though.






