Sputins
Member
- Joined
- Oct 28, 2014
- Messages
- 20
Okay so let’s “image” these Silver Crystals even further!
I know someone who knows someone with access to a million dollar “Scanning Electron Microscope”.
So I asked my someone and they sent the crystals in to the SEM team. The SEM people loved the Silver Crystals and took glee in checking them out. These are some preliminary pictures of the actual crystals as seen in the beaker from the previous post.
Starting out at 1mm scale and going through and down to 5um, four scales in the series of pictures.
Each scale has two images, the ETD which is the standard electron scan and the BSTD which is called the back-scatter scan. With the silver crystal, it shows different contrasts.
1mm ETD
1mm BSTD
500uM ETD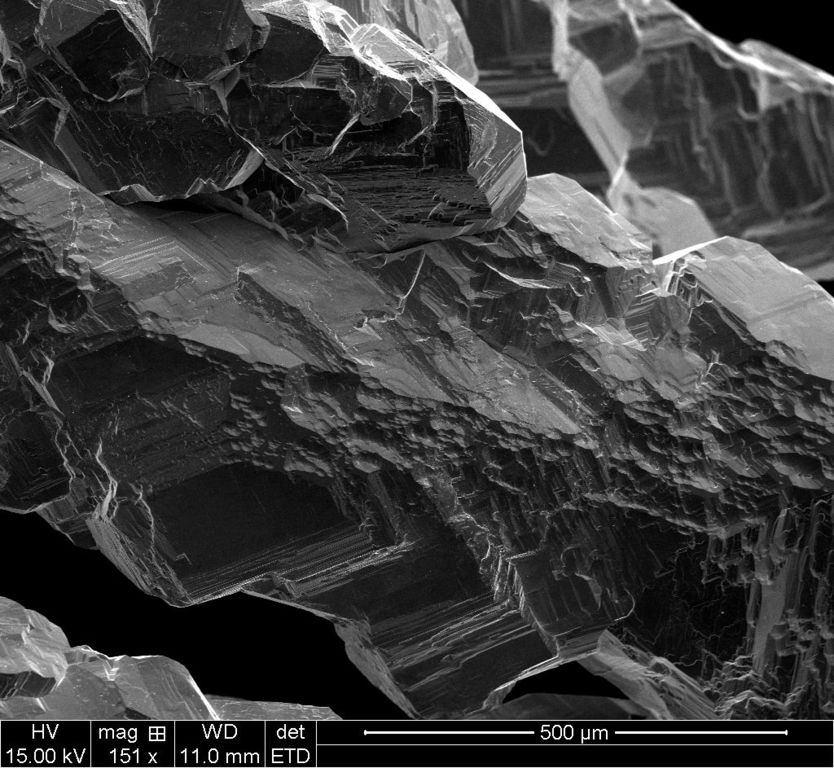
500um BSED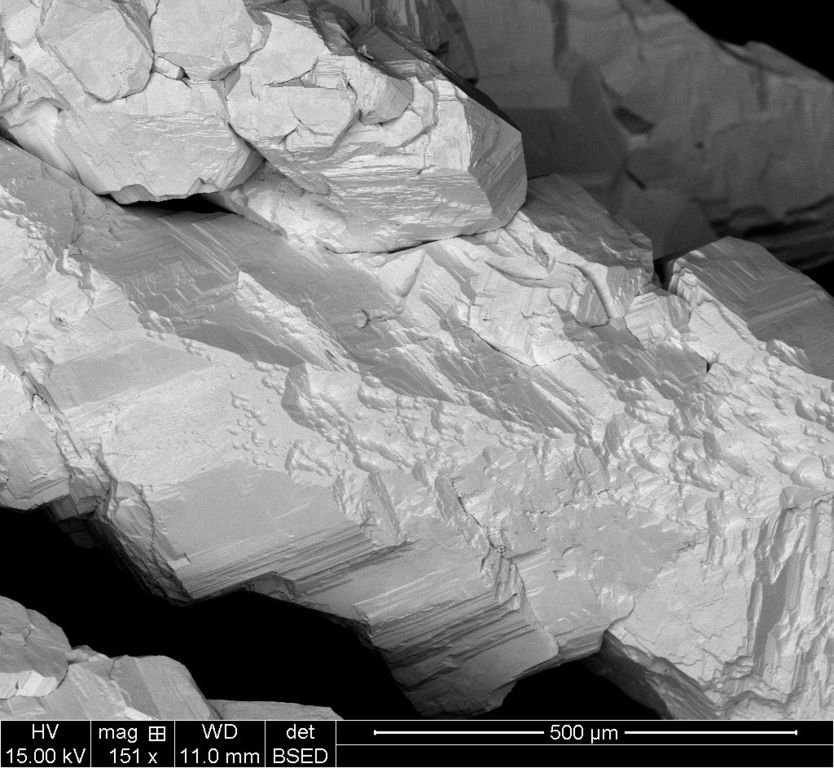
30um ETD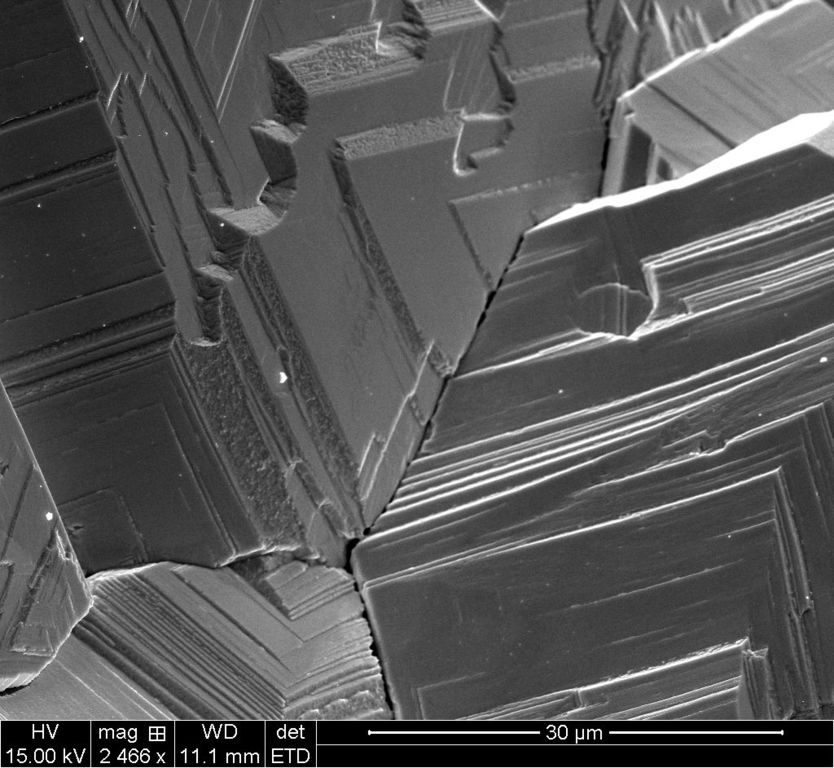
30um BSTD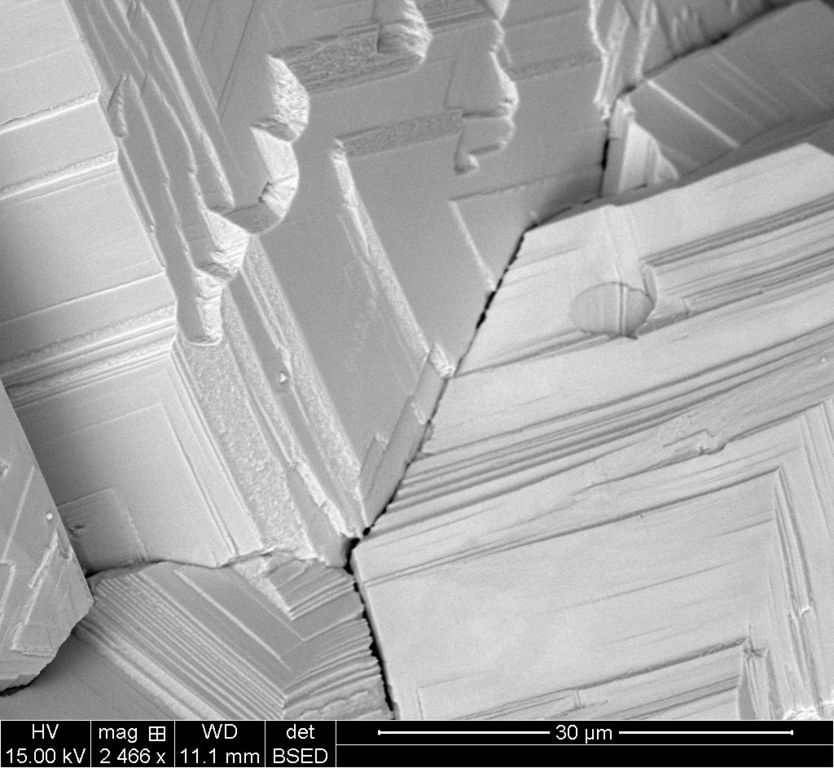
5um ETD
5um BSTD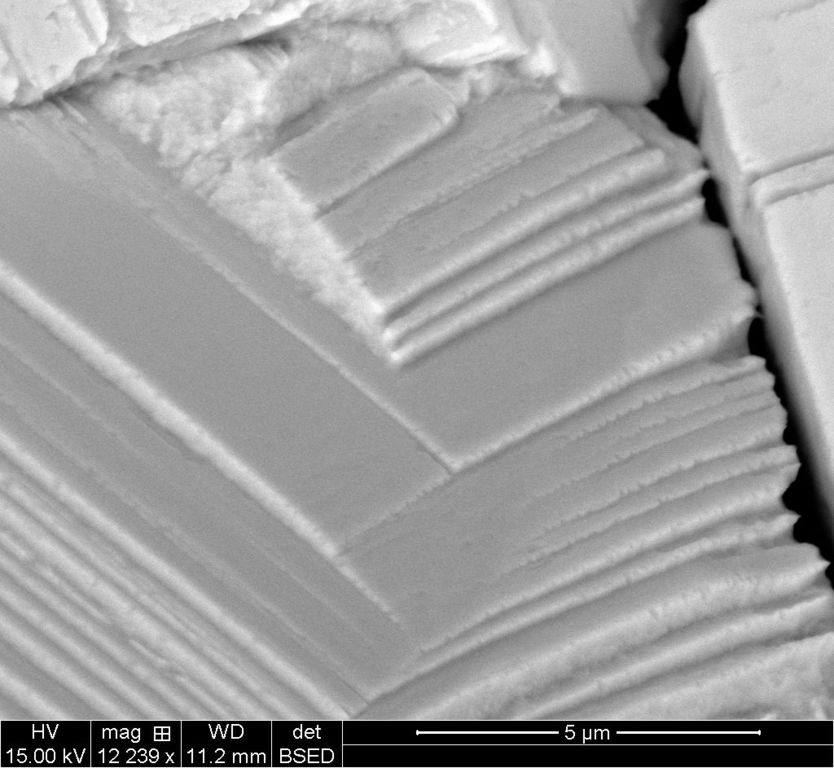
These scans were an initial 5 minute play and I’m told they’ll take have another more detailed in depth look at them.
I know someone who knows someone with access to a million dollar “Scanning Electron Microscope”.
So I asked my someone and they sent the crystals in to the SEM team. The SEM people loved the Silver Crystals and took glee in checking them out. These are some preliminary pictures of the actual crystals as seen in the beaker from the previous post.
Starting out at 1mm scale and going through and down to 5um, four scales in the series of pictures.
Each scale has two images, the ETD which is the standard electron scan and the BSTD which is called the back-scatter scan. With the silver crystal, it shows different contrasts.
1mm ETD

1mm BSTD

500uM ETD

500um BSED

30um ETD

30um BSTD

5um ETD

5um BSTD

These scans were an initial 5 minute play and I’m told they’ll take have another more detailed in depth look at them.

















































