takenbyvultures
Active member
- Joined
- Dec 4, 2011
- Messages
- 29
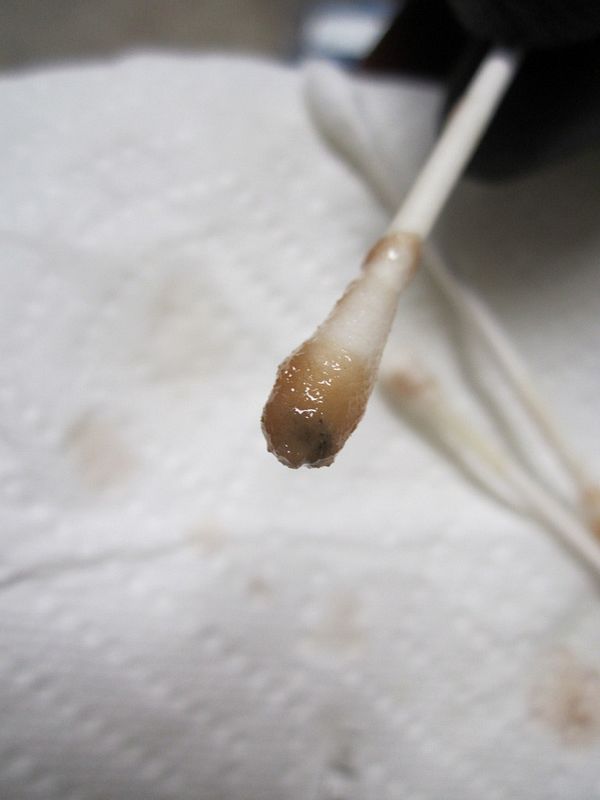
I've done 4 batches and every time I do the stannous test, i get this same color. Is this a false positive or bad stannous? I purchased the stannous in 1/2oz vial from ebay seller sparky112 and keep it in a cool dark place.