-
Please join our new sister site dedicated to discussion of gold, silver, platinum, copper and palladium bar, coin, jewelry collecting/investing/storing/selling/buying. It would be greatly appreciated if you joined and help add a few new topics for new people to engage in.
Bullion.Forum
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Electrochemistry Copper Nitrate Cell
- Thread starter rusty
- Start date

Help Support Gold Refining Forum:
This site may earn a commission from merchant affiliate
links, including eBay, Amazon, and others.
Jonn,
It is still sitting here by the computer table, I pick it up and admire it now and then, and Keep thinking about setting up my own small copper cell, to recover some of my waste for the fun of it, No I have not tried any tests, I do not think it would show much, the density, and thickness of the plating on this cathode copper kind of gives me an indication the copper is fairly pure, the color under the surface also indicates this. As I said before I am totally amazed you electro-winned this from an impure copper anode, your cathode copper came out beautiful.
I do not want to mess up this cathode, but would like to see how it would bend, I keep thinking the copper is so dense in the plating you could form it into shapes, just as you could pure melted copper (but for now I like it here just as it is, as a nice paper weight, it is nice thanks).
I still think you should make some posts on how you came up with these results, giving details.
It is still sitting here by the computer table, I pick it up and admire it now and then, and Keep thinking about setting up my own small copper cell, to recover some of my waste for the fun of it, No I have not tried any tests, I do not think it would show much, the density, and thickness of the plating on this cathode copper kind of gives me an indication the copper is fairly pure, the color under the surface also indicates this. As I said before I am totally amazed you electro-winned this from an impure copper anode, your cathode copper came out beautiful.
I do not want to mess up this cathode, but would like to see how it would bend, I keep thinking the copper is so dense in the plating you could form it into shapes, just as you could pure melted copper (but for now I like it here just as it is, as a nice paper weight, it is nice thanks).
I still think you should make some posts on how you came up with these results, giving details.
Thank you Buthcher, that means a lot coming from you. There is actually a way to solid plate onto or into any shapes. The plating process requires patience as it takes a while for sure, but it is well worth it. The purity is high according to the xrf, shows 999. I run multiple cathodes and that makes it worth while as it gives more weight in the same amount of time. The batch I ran your sample in was calculated at .07 cents worth of electricity. Much cheaper than melting and cleaner too.
I will post details and pictures soon, if you think it's a good idea. I'm no pro, far from it. I appreciate the compliment. I will also post the process of making any shapes. Let me know when you get your power supply, rectifier, working. Always, good to hear from you, Jonn.
I will post details and pictures soon, if you think it's a good idea. I'm no pro, far from it. I appreciate the compliment. I will also post the process of making any shapes. Let me know when you get your power supply, rectifier, working. Always, good to hear from you, Jonn.
Jonn,
I believe a lot of members, myself included, would be interested in your process. Please do share!
Thanks,
Dave
I believe a lot of members, myself included, would be interested in your process. Please do share!
Thanks,
Dave
Thank you for the kind words Dave. It has been proven through my experience this past year that copper likes to plate out in solid form at the proper voltage. There are many charts available online and in books, all stating the same fact. The voltage for copper is .34
The process is quite simple really, melt an anode, blister copper is what it's called. It may have a few percent impurities with the balance copper. What seems to work best is 97% copper. That should not be hard to accomplish considering all the copper in electronics and ewaste. The balance or 3% would contain silver, gold, palladium or whatever else is in your scrappile. I purposely add 30% by weight additional copper. This may be reclaimed from your solutions from fingers or foils or whatever else you may have copper in that needs to be dropped. That copper powder can be added to your blister anode to achieve your 97%. A lower percentage works but not as well. I strive for at least 93% copper minimum.
The anode of blister copper and the cathode are placed no more than 4" apart in spent nitric solutions used for eliminating base metals. Once again, this contains mostly copper. The cathode should have the same area as the anode for proper deposition. If my anode is 3" x 5" then my cathode is also 3" x 5". The cathode is a thin sheet of rolled copper from the rolling mill. ( pm me if you need some rolled sheet ). The voltage is strictly controlled not to exceed .4 volts preferably as close to .34 volts as possible with a slight excess only. Once you go above .8 volts, you will plate silver.
A good source for this anode material may include silver plated copper tableware.
I run more than one anode and cathode at the same time in a 5 gallon bucket.
I fashion a square lid for the round bucket top. In that lid I scribe a radius 4" for an 8" diameter mark. Along that mark I drill every 4" a 1/4" hole. Mine has 6 holes in it. Through every other hole I looped a #8 stranded copper wire with each end meeting on top of the lid in the center. These ends are twisted together and my negative clip attaches here.
The cathode copper starter sheets are rolled at top about 3/8" to hang on this #8 wire. This roll serves 2 purposes,
1. It makes contact with my negative voltage
2. It shortens the cathode a bit, the bottom is the strongest point of attachment and should be slightly higher than the anode in the electrolyte.
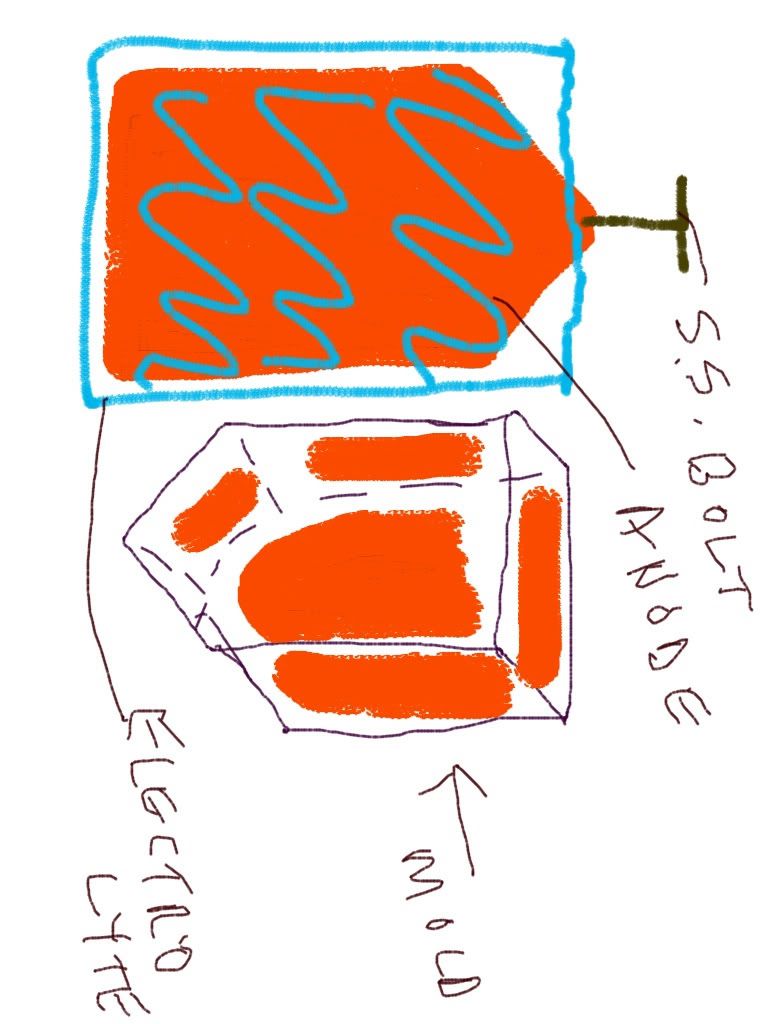
The anode holes get a drill hole in the top and tapped for a #8 stainless steel bolt with washer on top of the lid.
Take the positive wire and make 3 ends or pigtails. Crimp a round yellow terminal to each end. Insert the stainless screw and washer through this terminal. Insert this into your lid in every other hole. Thread this bolt into the top of your 3 anodes. Turn on your power source with the voltage all the way down. Immerse your plate with cathodes and anodes into your spent nitric electrolyte. Immediately adjust your voltage to .34 or just slightly higher. The amperage will adjust itself according to the size of your elctrodes and spacing. Slight agitation daily is recommended. This will take a while. It may work pretty much undisturbed except for the daily agitation.
The copper cathode is very pure. It is hard as in pure copper sheet. It cannot be knocked off even with a hammer. It can be rolled in the mill after washing or recycled for top dollar. It is pure metal. I get #1 pricing. This is what's indeed called a grade A cathode, this is what spot price of copper is based on.
Butcher, could you post post a picture of the one you have? I sold all mine.
The process is quite simple really, melt an anode, blister copper is what it's called. It may have a few percent impurities with the balance copper. What seems to work best is 97% copper. That should not be hard to accomplish considering all the copper in electronics and ewaste. The balance or 3% would contain silver, gold, palladium or whatever else is in your scrappile. I purposely add 30% by weight additional copper. This may be reclaimed from your solutions from fingers or foils or whatever else you may have copper in that needs to be dropped. That copper powder can be added to your blister anode to achieve your 97%. A lower percentage works but not as well. I strive for at least 93% copper minimum.
The anode of blister copper and the cathode are placed no more than 4" apart in spent nitric solutions used for eliminating base metals. Once again, this contains mostly copper. The cathode should have the same area as the anode for proper deposition. If my anode is 3" x 5" then my cathode is also 3" x 5". The cathode is a thin sheet of rolled copper from the rolling mill. ( pm me if you need some rolled sheet ). The voltage is strictly controlled not to exceed .4 volts preferably as close to .34 volts as possible with a slight excess only. Once you go above .8 volts, you will plate silver.
A good source for this anode material may include silver plated copper tableware.
I run more than one anode and cathode at the same time in a 5 gallon bucket.
I fashion a square lid for the round bucket top. In that lid I scribe a radius 4" for an 8" diameter mark. Along that mark I drill every 4" a 1/4" hole. Mine has 6 holes in it. Through every other hole I looped a #8 stranded copper wire with each end meeting on top of the lid in the center. These ends are twisted together and my negative clip attaches here.
The cathode copper starter sheets are rolled at top about 3/8" to hang on this #8 wire. This roll serves 2 purposes,
1. It makes contact with my negative voltage
2. It shortens the cathode a bit, the bottom is the strongest point of attachment and should be slightly higher than the anode in the electrolyte.
The anode holes get a drill hole in the top and tapped for a #8 stainless steel bolt with washer on top of the lid.
Take the positive wire and make 3 ends or pigtails. Crimp a round yellow terminal to each end. Insert the stainless screw and washer through this terminal. Insert this into your lid in every other hole. Thread this bolt into the top of your 3 anodes. Turn on your power source with the voltage all the way down. Immerse your plate with cathodes and anodes into your spent nitric electrolyte. Immediately adjust your voltage to .34 or just slightly higher. The amperage will adjust itself according to the size of your elctrodes and spacing. Slight agitation daily is recommended. This will take a while. It may work pretty much undisturbed except for the daily agitation.
The copper cathode is very pure. It is hard as in pure copper sheet. It cannot be knocked off even with a hammer. It can be rolled in the mill after washing or recycled for top dollar. It is pure metal. I get #1 pricing. This is what's indeed called a grade A cathode, this is what spot price of copper is based on.
Butcher, could you post post a picture of the one you have? I sold all mine.
Jonn,
Thanks for taking the time to write up the details of your setup. I'll probably be trying this in the spring.
How are you melting your copper feedstock to make your anodes? Are you using a furnace or torch melting in a melting dish? I've read that oxidation during the melt is a problem with copper. Are you experiencing this or are you minimizing it by melting with a cover or a carbon source?
Thanks,
Dave
Thanks for taking the time to write up the details of your setup. I'll probably be trying this in the spring.
How are you melting your copper feedstock to make your anodes? Are you using a furnace or torch melting in a melting dish? I've read that oxidation during the melt is a problem with copper. Are you experiencing this or are you minimizing it by melting with a cover or a carbon source?
Thanks,
Dave

$19.99
$24.99
PAXCOO Jewelry Making Supplies Kit with Jewelry Tools, Jewelry Wires and Jewelry Findings for Jewelry Repair and Beading
Paxcoo Direct

$84.83
$171.00
Catalysis in the Refining of Fischer-Tropsch Syncrude (Catalysis Series, Volume 4)
Basi6 International

$22.59 ($3.23 / Count)
$23.99 ($3.43 / Count)
WORKPRO 7-Piece Jewelers Pliers Set, Jewelry Making Tools Kit with Easy Carrying Pouch (Blue)
GreatStar Tools

$15.07
$30.00
Refining Expertise: How Responsible Engineers Subvert Environmental Justice Challenges
Kuhns Corner Books
Geo
Well-known member
your copper anodes will only oxidize on the outer surface. use a wire brush and brush it till its shiny.
Geo is right, it only oxidizes on the outer surface, the surface exposed to air during the pour. I melt mine with minimal flux in a furnace. I pour the anodes into a core of sorts. I will explain, the bottom of the mold as well as the sides, get completely filled with molten copper. Oxidation is minimal in those areas. The top does oxidize a bit but it has caused no problems for me. Once cool, the anode is removed and used upside down. The oxidized portion being at the bottom. My mold is tapered at the bottom to a point. Once cooled, this point becomes the top of the anode. As the anode dissolves, it leaves less material because of the pointed top. A small portion of the top is left above the electrolyte, about 1/2". Hope this helps, Jonn
John said: "The copper cathode is very pure. It is hard as in pure copper sheet. It cannot be knocked off even with a hammer. It can be rolled in the mill after washing or recycled for top dollar. It is pure metal."
Some years ago I electroformed some heat exchanger parts using copper sulfate solution. That copper was the hardest I've ever seen. Unfortunately my cathodes were not smooth and grew some polyps. What I would do is cut off the polyps and hammer to rehape my piece as necessary, but first I had to get the copper soft (temper it) by heating and quenching it in water.
I have two questions:
1. Are all sucessful solid and thick electro deposits naturally hard?
2. How can you roll copper that is hard? Is there a tempering process somewhere you haven't mentioned? Most copper sheet that one buys comes only partially hard.
Very interesting thread,
FrugalEE
Some years ago I electroformed some heat exchanger parts using copper sulfate solution. That copper was the hardest I've ever seen. Unfortunately my cathodes were not smooth and grew some polyps. What I would do is cut off the polyps and hammer to rehape my piece as necessary, but first I had to get the copper soft (temper it) by heating and quenching it in water.
I have two questions:
1. Are all sucessful solid and thick electro deposits naturally hard?
2. How can you roll copper that is hard? Is there a tempering process somewhere you haven't mentioned? Most copper sheet that one buys comes only partially hard.
Very interesting thread,
FrugalEE
Hi frugalEE, the copper is as hard as a copper pipe or copper wire. I simply meant that it is pure solid metal, not fluff or sponge or powder. It can be hammered or rolled just like any other pure copper. I noticed growths of polyps as well if not agitated. Those would be from any dust or spec of scum that has attached to the cathode during the process. I like to agitate daily and every once in a while, I would take a horse syringe, pull some electrolyte through it and spray that directly onto each side of each cathode. In essence washing off anything that stuck and agitating at the same time.
It has been said, although I haven't tried this yet, that if you could surge the voltage purposely, it would knock off anything that applies itself to the cathode. Maybe a relay and timer could be connected Inline for that purpose? Just a thought.
It has been said, although I haven't tried this yet, that if you could surge the voltage purposely, it would knock off anything that applies itself to the cathode. Maybe a relay and timer could be connected Inline for that purpose? Just a thought.
Thanks John,
I used the word temper in my previous post when I should have said anneal. Annealing is using heat to soften. Copper wire is about the purest form of copper readily available and it usually comes pretty soft so it will bend. I can flatten some 12 gauge house wire up to about 3 times its initial diameter producing almost a knife blade like hardness, but without annealing I really can't get it much thinner. The copper I electroformed was hard like that hammered copper and I expect your's was too. I only know of two ways to make copper harder - work it or electrodeposit it. I still doubt that you would be able to roll your copper cathode directly very succesfully without first annealing it. I suspect copper is like gold in that it doesn't take much of some kinds of impurities to make the metal brittle and resistant to rolling, hence rolling is a test of purity. Perhaps very pure electrodeposited copper can be rolled, at least with high pressures. Also I'm wondering if it's possible in some way to electro-deposit copper with a softer temper. Perhaps your nitric deposits are softer. I did some nitric deposits and my twig like pieces are very hard and brittle.
It wouldn't take much to have a relay periodically reverse polarity or insert another power source to change voltage. I've read somewhere that things like that can be used to reduce the amount of baby sitting needed and improve deposits.
Not many have produced at bar like you have. It takes a lot of patience.
FrugalEE
I used the word temper in my previous post when I should have said anneal. Annealing is using heat to soften. Copper wire is about the purest form of copper readily available and it usually comes pretty soft so it will bend. I can flatten some 12 gauge house wire up to about 3 times its initial diameter producing almost a knife blade like hardness, but without annealing I really can't get it much thinner. The copper I electroformed was hard like that hammered copper and I expect your's was too. I only know of two ways to make copper harder - work it or electrodeposit it. I still doubt that you would be able to roll your copper cathode directly very succesfully without first annealing it. I suspect copper is like gold in that it doesn't take much of some kinds of impurities to make the metal brittle and resistant to rolling, hence rolling is a test of purity. Perhaps very pure electrodeposited copper can be rolled, at least with high pressures. Also I'm wondering if it's possible in some way to electro-deposit copper with a softer temper. Perhaps your nitric deposits are softer. I did some nitric deposits and my twig like pieces are very hard and brittle.
It wouldn't take much to have a relay periodically reverse polarity or insert another power source to change voltage. I've read somewhere that things like that can be used to reduce the amount of baby sitting needed and improve deposits.
Not many have produced at bar like you have. It takes a lot of patience.
FrugalEE
Thank you frugalEE, it does take patience for sure. But it's a nice finished useable product and the slimes can be processed for values. The electrolyte would otherwise not be used and the cost is minimal. All good reasons to be patient. I found a cathode I ran a while back sitting in my drawer, I will post a picture and then roll it and post a picture of that. I believe when rolling any material annealing is important if you intend to use the rolled product. If you roll thin and curl your sheet for parting it's not as important but still a good idea. Thanks, Jonn.
The words temper and anneal are often misused. In general usage tempering something means only to change it by the addition of something.
I have done a fair share of blacksmithing and in the iron mongering world you temper something by adding heat, then quenching it cold rapidly to save that state of the iron. This makes an iron alloy in its hardest most brittle state. You then anneal the iron by controlled heat to soften it to the desired “temper”.
Copper is very different in that to achieve the softest state of the material you heat it cherry red then quench it rapidly to achieve the most ductile and softest state.
I have done a fair share of blacksmithing and in the iron mongering world you temper something by adding heat, then quenching it cold rapidly to save that state of the iron. This makes an iron alloy in its hardest most brittle state. You then anneal the iron by controlled heat to soften it to the desired “temper”.
Copper is very different in that to achieve the softest state of the material you heat it cherry red then quench it rapidly to achieve the most ductile and softest state.
John/Butcher - first I want to thank the both of you for providing the info here on running a copper/nitrate cell & electro chemistry in general as I am planing to attempt this process in the near future.
I have read & re-read the last 2 pages of this thread 5 or 6 times now as well as looked at the links provided & I have a number of questions
I will start with a question about tin in the mix (of the anode)
My anodes will be made up of metals smelted from incinerated high grade circuit boards such as hard drive boards, cell phone boards, ram, slot processors & chips - so tin will be a high % and I understand I may need to ad extra copper to get my anodes up to at least 93% or better copper
The question is - how does the tin "report" in this process --- another words - in theory everything below copper in the reactive serries should report as anode slime & everything above copper should go into solution (the electrolite)
However - we are using a nitrate solution as our electrolite & as we know nitric acid does not dissolve tin but turns it to tin paste (or stannic tin) so does the tin report in the anode slime & if so is that report of tin in the form metalic tin or stannic tin?
My "assumption" is that because the electrolite is a pregnant copper nitrate (or near pregnant) that the tin would report as tin metal with some residual stannic tin ?
Kurt
I have read & re-read the last 2 pages of this thread 5 or 6 times now as well as looked at the links provided & I have a number of questions
I will start with a question about tin in the mix (of the anode)
My anodes will be made up of metals smelted from incinerated high grade circuit boards such as hard drive boards, cell phone boards, ram, slot processors & chips - so tin will be a high % and I understand I may need to ad extra copper to get my anodes up to at least 93% or better copper
The question is - how does the tin "report" in this process --- another words - in theory everything below copper in the reactive serries should report as anode slime & everything above copper should go into solution (the electrolite)
However - we are using a nitrate solution as our electrolite & as we know nitric acid does not dissolve tin but turns it to tin paste (or stannic tin) so does the tin report in the anode slime & if so is that report of tin in the form metalic tin or stannic tin?
My "assumption" is that because the electrolite is a pregnant copper nitrate (or near pregnant) that the tin would report as tin metal with some residual stannic tin ?
Kurt
Kurt,
I cannot answer your question; here are a few my thoughts.
Tin in HNO3 does not really dissolve, the reason I believe is because the nitric is such a strong oxidizer, and tin so reactive, the tin just oxidizes, and since it does not dissolve into solution it will not pass through filters worth a darn.
so in the nitrate solution you may be able to keep some of the metastannic acid in the anode bag,
But the more you could remove before your made copper anode, I think the less trouble in your cell, I would not only look at adding copper to raising percentage, but would look into pretreatments to removing the other metals first. Like tin iron and the other base metals, with chemical and furnace treatments.
Copper is normally refined in copper sulfate cells, my guess is there must be a reason, they choose the sulfate over the nitrate electrolyte.
Tin as I understand it in these cell can partially be reduced on cathode as tin salts if the percentage of tin is too high, so I believe when electrolyte begins to become contaminated they remove a portion of electrolyte to clean it up, or replace electrolyte.
I think when they refine copper first they refine the copper in reverberatory furnaces, in an oxidizing melt, sometimes blowing in oxygen to the melt, to oxidize metals like iron, lead and tin, forming a slag, which they remove many of the oxidized base metals from the copper.
To me this sounds hard as copper can also oxidize fairly easy in a melt, so it would seem kind of art to oxidizing unwanted metal without oxidizing the copper.
I cannot answer your question; here are a few my thoughts.
Tin in HNO3 does not really dissolve, the reason I believe is because the nitric is such a strong oxidizer, and tin so reactive, the tin just oxidizes, and since it does not dissolve into solution it will not pass through filters worth a darn.
so in the nitrate solution you may be able to keep some of the metastannic acid in the anode bag,
But the more you could remove before your made copper anode, I think the less trouble in your cell, I would not only look at adding copper to raising percentage, but would look into pretreatments to removing the other metals first. Like tin iron and the other base metals, with chemical and furnace treatments.
Copper is normally refined in copper sulfate cells, my guess is there must be a reason, they choose the sulfate over the nitrate electrolyte.
Tin as I understand it in these cell can partially be reduced on cathode as tin salts if the percentage of tin is too high, so I believe when electrolyte begins to become contaminated they remove a portion of electrolyte to clean it up, or replace electrolyte.
I think when they refine copper first they refine the copper in reverberatory furnaces, in an oxidizing melt, sometimes blowing in oxygen to the melt, to oxidize metals like iron, lead and tin, forming a slag, which they remove many of the oxidized base metals from the copper.
To me this sounds hard as copper can also oxidize fairly easy in a melt, so it would seem kind of art to oxidizing unwanted metal without oxidizing the copper.
Butcher is right on all accounts. Steel would be removed first by hand, tin would oxidize and partially volatise in the melting of the anode. The flux would would remove most if not all of the tin. Any tin left would report to the slimes. Also be sure to rejuvenate the electrolyte periodically. Don't ever turn off your power supply before removing your anode and cathode, otherwise you will coat your cathode with silver as in the reactivity displacement similar to cementing. I like to spray mine off into the electrolyte before removing them. This dirty solution can become your winning cell once all slimes are removed. You would use either hard graphite sheet or titanium as the anode and a copper starter sheet for your cathode. The voltage would have to be increased. Pm me if you need graphite or titanium.
The winning cell would remove most of the junk from your electrolyte in turn rejuvenating your solution. Eventually you would send this electrolyte to your waste cleanup,(see elephant in the room.) hope this helps, Jonn
The winning cell would remove most of the junk from your electrolyte in turn rejuvenating your solution. Eventually you would send this electrolyte to your waste cleanup,(see elephant in the room.) hope this helps, Jonn
Butcher/John – thanks for the replies
John – what are you using to help flux out the tin ? ---I ask because a while back as an experiment I incinerated a hard drive board & then smelted it & the resulting button was still high in tin – enough tin to give the button a pinkish color
I used soda ash as my flux – no borax as I figured the glass from the fiberglass would take the place of borax
Are you using something like ferric oxide or potassium nitrate as an oxidizing agent in your flux ?
Also I plan to order some fluorspar to use as a thinning agent in the flux to help with settling out of the metals from the slag
Kurt
John – what are you using to help flux out the tin ? ---I ask because a while back as an experiment I incinerated a hard drive board & then smelted it & the resulting button was still high in tin – enough tin to give the button a pinkish color
I used soda ash as my flux – no borax as I figured the glass from the fiberglass would take the place of borax
Are you using something like ferric oxide or potassium nitrate as an oxidizing agent in your flux ?
Also I plan to order some fluorspar to use as a thinning agent in the flux to help with settling out of the metals from the slag
Kurt
Butcher
You wrote - Copper is normally refined in copper sulfate cells, my guess is there must be a reason, they choose the sulfate over the nitrate electrolyte.
Yes I understand this & it is especially true of copper refined from ores & as I understand it that is in part due to the nature of ores to start with
What sparked my interest in this thread back when Rusty first started it was not the idea of refining copper but rather the idea of dealing with the copper in the process of recovering the PMs for refining were in copper makes up a large part of the base metal to be dealt with
Leaching of course is one way of dealing with the base metals – but leaching presents its own set of problems – one of which is the waste generated & copper nitrate is a waste we generate in many of our process
That is what has sparked my interest in this thread – being able to utilize waste generated from other processing to deal with the copper that would other wise require more leaching which would in turn generate more waste
So ultimately the goal is not so much to refine copper – but rather recover (& then refine) PMs from “high” copper content material that utilizes waste generated (copper nitrate) in order to reduce generating more waste (more copper nitrate) by leaching
There is of course an up side & down side to each method (leaching or electrolytic)
Leaching = faster – but more waste
Electrolytic = less waste – but slower
Comments welcome
Kurt
You wrote - Copper is normally refined in copper sulfate cells, my guess is there must be a reason, they choose the sulfate over the nitrate electrolyte.
Yes I understand this & it is especially true of copper refined from ores & as I understand it that is in part due to the nature of ores to start with
What sparked my interest in this thread back when Rusty first started it was not the idea of refining copper but rather the idea of dealing with the copper in the process of recovering the PMs for refining were in copper makes up a large part of the base metal to be dealt with
Leaching of course is one way of dealing with the base metals – but leaching presents its own set of problems – one of which is the waste generated & copper nitrate is a waste we generate in many of our process
That is what has sparked my interest in this thread – being able to utilize waste generated from other processing to deal with the copper that would other wise require more leaching which would in turn generate more waste
So ultimately the goal is not so much to refine copper – but rather recover (& then refine) PMs from “high” copper content material that utilizes waste generated (copper nitrate) in order to reduce generating more waste (more copper nitrate) by leaching
There is of course an up side & down side to each method (leaching or electrolytic)
Leaching = faster – but more waste
Electrolytic = less waste – but slower
Comments welcome
Kurt