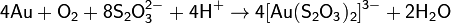solar_plasma
Well-known member
freechemist said:Correct equation for precipitation of silver sulfide:
2 [Ag(S2O3)2]3- + S2- ==> Ag2S + 4 S2O32-
If the S2- would be added by a solution of alkali sulfide like K2S, would we be left with a solution of alkali, chloride and thiosulfate ions, so we could reuse this solution for the same purpose?
edit: if so, the silver sulfide could be used as SO2 source for the precipitation of gold... :?: :idea: