I've been thinking in similar tracks for some years now, how to remove the gold from nitric acid without destroying the nitric acid in the process...
This is so far a purely theoretical solution. I haven't had the opportunity to test my theory yet.
The clue is to get rid of all chloride ions so the gold doesn't redissolve. As long as you have gold chloride or other soluble chlorides there exists free chloride ions in solution and with the nitric acid it can be seen as free hydrochloric acid.
Now, we have one easily acquired chemical that removes virtually all free chloride ions from a solution, silver nitrate. If we mix silver nitrate into our leach until no more silver chloride is formed then we have a chloride free gold nitrate solution.
From this we can drop the gold with SMB, SO
2 or possibly even electrowinning.
When the gold is gone from the solution the only thing left to do is to add a bit of hydrochloric acid again and then continue to use the reverse aqua regia.
Using SMB / SO2 will probably add small amounts of sulfuric acid to the mix and that could be a problem with nitric acid. Electrowinning the gold would not add any contaminants.
Anyone sees a problem with this process?
The gold would probably need to be refined again to remove palladium if SMB / SO
2 is used. Electrowinning will probably also give a mixed product of gold, silver and copper.
As a byproduct this would produce a solution with both gold and silver in solution... and whatever Harold says I think there is another way to have both silver and gold in solution besides using cyanide. :mrgreen:
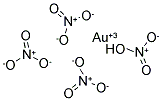
Meet
hydrogen tetranitroaurate(III)
Göran





![WP_20151008_03_32_21_Pro[1].jpg](https://cdn.imagearchive.com/goldrefiningforum/data/attachments/20/20781-d26aa63235739764d164a8b5b2726fc3.jpg)